

ABSTRACT: Forty-two years ago (King 1978) I proposed the biocosmological thesis that the form of life's origin and evolution is a cosmological interactive process defined in the cosmic symmetry-breaking of the four forces of nature at the origin of the universe. With the passage of time, the pendulum has shifted from the improbability of life as a random molecular accident to an awareness that central biomolecules may be cosmologically abundant products of the clouds forming young stars leading to an RNA-era in which both catalysis and replication emerged from one cosmologically dervied molecule RNA. This paper unveils the non-linear quantum foundations of biocosmology as the founding science of life.
The Signature Fingerprint of the First Life on Earth - an overview of the latest perspectives.
Keywords : cosmology symmetry-breaking, molecular evolution, chaos, complex system, neurodynamics, quantum non-locality, transaction, consciousness.
Fig 1: Paradise on the Cosmic Equator: Darwin, the serpent and the hoopoe in Eden: Biological systems form a central cosmological manifestation of interactive complexity in the universe. When space-time is considered as a 4-manifold, biology's 'equatorial' position is as fundamental in cosmological terms, even though biological energetics are too weak to withstand either the polar big-bang at the origin nor the possible final fates, whether they are heat death by attrition in an ever-expanding universe, a big crunch, or fractal inflation, as shown at the left.
Could biological structures such as tissues, and organisms be cosmological interactive structures as fundamental as stars and galaxies to the cosmic design? The conventional objections are obvious. Life is a fragile insignificance among the immense energies of black holes, galaxy formation and the big-bang. Its tiny entropy-reducing photosynthetic energy budget and fragile chemical bonds are insignificant on the cosmic scale. Biological structures are genetically coded in a vast variety of ways by specific nucleic acid sequences. Biological evolution is a stochastic process combining random mutation and selective advantage, many of whose manifestations are opportunistic. Nevertheless many features of life as we know it on Earth may be the product of cosmic factors determining the laws of nature which make life possible.
Although traditional chemistry, despite its quantum foundations, treats molecules as arbitrary building blocks which can be arranged in almost any combination using suitable reagents and conditions, there is clear evidence for optimality of many prebiotic and biological molecules, giving life as we know it a cosmological basis as a culminating interactive structure.
This paper explains how and why the origins of chemical life, major aspects of biological evolution and the elaborate emergent structures of tissues, from biomolecules up to cellular organelles and even to the doors of perception of the conscious brain, are a fractal interactive consequence of the non-linear laws of nature established at the cosmic origin. This reverses the Copernican revolution, putting life and with it ourselves back to centre stage in the cosmic arena.
Biology is a product of the twisted laws of nature dervied from cosmic symmetry-breaking. The rich diversity of structure in molecular systems is made possible by the profound asymmetries developing at the cosmic origin, between the nuclear forces, gravity and electromagnetism. The diversity of the elements and their asymmetric charge structure, with clusters of negatively charged electrons orbiting a massive nucleus containing all the positive charges in a concentrated nuclear 'droplet', is made possible only through the divergence of symmetry of the four fundamental forces. Without these asymmetries there would be only one or two simple atoms and none of the richness of the almost unlimited variety of molecular structures which can be generated by the over one hundred complex atoms occurring in nature as we know it. Chemical bonding is a consequence of the non-linear inverse square law of electromagnetic charge interaction in space-time. This non-linearity also gives rise to a succession of weak bonding interactions, generating the complex non-periodic secondary and tertiary structures of proteins and nucleic acids.
 Fig 2: Galaxies and galaxy clusters illustrate the fractal self-similar nature of large scale fluctuations in the universe. According to some versions of inflation theory these may be inflated quantum fluctuations. Left: Galaxy MC 100. Right: a distant gravitationally-lensed red galaxy beyond a closer cluster, including blue galaxy lover right. Top right inset large scale structure of the universe including the 'great wall'.
Fig 2: Galaxies and galaxy clusters illustrate the fractal self-similar nature of large scale fluctuations in the universe. According to some versions of inflation theory these may be inflated quantum fluctuations. Left: Galaxy MC 100. Right: a distant gravitationally-lensed red galaxy beyond a closer cluster, including blue galaxy lover right. Top right inset large scale structure of the universe including the 'great wall'.GENERATING A COMPLEX, TWISTED UNIVERSE
The four fundamental forces of nature - the strong and weak forces mediating nuclear binding and neutron decay respectively, along with electromagnetism and gravity are believed to have emerged from a single superforce, perhaps a form of higher-dimensional string, or membrane theory, in twelve, or so dimensions, immediately after the big bang, fig 3(a). The higher-dimensional space, containing a single generalized superforce compactified most of its dimensions to sub-particulate scales, leaving the four dimensions of space-time and broke symmetry to form the different forces we see today, in much the way a ferromagnet is polarized at minimum energy, breaking symmetry in space, so that at the lowest energy, all domains point in one direction. The forces nevertheless do appear to converge at extremely high energies - the unification temperature.
The strong force is a secondary effect of the colour force between the three red, green and blue quarks comprising a proton or neurtron in much the same way that molecular bonding is a secondary consequence of the formation of atoms. The colour force has three colours and three anti-colours instead of two charges. It also comes in two ground flavours so that the proton and neutron are a composite of up and down flavours uud and udd as well as three different colours. The quarks' charges of u = +2/3 and d = -1/3 thus generate precisely the integral charges of the proton and neutron. The weak force has become very short range because it is mediated by massive particles, which are believed to gain the required extra degree of freedom by assimilating another concealed particle, the mysterious Higgs boson (Georgi 1981, t'Hooft 1980, Veltman 1986) as eventually discovered in the LHC (Slezak 2012).
Complementing this picture at the quantum field theory level is a description on the cosmic scale in which a central theme is inflation. Although recently questioned by difficulties finding enough dark matter to halt the universe's slide towards hyperbolic expansion (Krauss 1999, Bucher and Spergel 1999), inflation concepts remain central to understanding how symmetry-breaking of the forces may have generated the expanding universe we know. In summary, a seed universe in the symmetrical state, below the unification temperature is in an unstable high-energy false vacuum, like a super-cooled liquid which could freeze to form a polarized magnet. The false vacuum in the Higg's field causes a gravitational repulsion representing the negative energy difference between temperature and that required to maintain the Higgs field. Under this 'antigravity', the empty universe, expands exponentially, smoothing quantum irregularities to structures on the scale of galaxies (Guth & Steinhardt 1984). The breakdown of the false vacuum (in 10-39 sec) halts this inflationary phase, releasing a shower of high-energy particles as latent heat, forming the hot expanding universe under attractive gravitation we are familiar with. The gravitational potential energy gained almost exactly equals the kinetic energy of the particles, making the generation of the universe possible from a quantum fluctuation. Indications are that the universe will continue to expand suggesting a hyperbolic inflation or fractal cosmic inflation (Linde 1992), in which the active tips of the universe are permanently inflating, to leave behind non-inflating bubble universes such as ours.
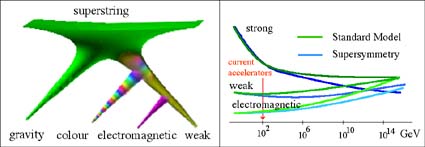 Fig 3 (a) Divergence of the four forces from a single superforce. (b) The three non-gravity forces converge in strength at the unification temperature.
Fig 3 (a) Divergence of the four forces from a single superforce. (b) The three non-gravity forces converge in strength at the unification temperature.What interests us here are the interactive consequences of this symmetry-breaking differentiation, because it leads to all the complex structures we see around us today. Cosmology is traditionally pre-occupied with alpha and omega - initial and final causes - the origin and fate of the universe. But there is another perspective in which life and its complexity is as central to cosmology, fomring the central non-linear interactive processes that make the universe the complex one we know and exist within, during the vast epochs of its mature evolution.
Although life may be created and annihilated during the evolution of the universe from alpha to omega, just as the creation and anihilation of virtual particles are essential to quantum field theory, the biological forms and processes can have a cosmic origin as generic structures and a cosmic significance as culminating interactive complexity (fig 1). Although fragile, on the cosmic scale of energies, the complexity of life is the supreme culmination in complexity of the interactive quantum process initiated in the quantum symmetry-breaking.
 Fig 4: The standard model of particle physics involves half-integer spin fermions which obey the Pauli exclusion principle and form matter and integer spin bosons which mediate force and radiation. Right: the composite structure of symmetry-broken fermionic mattter is molecular.
Fig 4: The standard model of particle physics involves half-integer spin fermions which obey the Pauli exclusion principle and form matter and integer spin bosons which mediate force and radiation. Right: the composite structure of symmetry-broken fermionic mattter is molecular.The interaction between the wave-particles emerging from the cosmic origin results in distinct effects on microscopic and cosmic scales. On the cosmic scale we find fractal structures - galaxy clusters, star and planetary formation, mediated by gravity, through contraction, heating and the ignition of the strong nuclear force, producing the energy of stars and the secondary photosynthetic energy of visible light.
 Fig 4a: The Crab Nebula, a supernova noted by Chinese astronomers in 1054.
Fig 4a: The Crab Nebula, a supernova noted by Chinese astronomers in 1054.
On the quantum scale we find integration of quarks to protons and neutrons then atomic nuclei in stars, then supernovas in the formation of chemical elements, and finally molecules, in the lower energetics of second generation sun-like stars. Quantum interaction of fermions reaches its full interactive complexity only in the molecular assemblies of biochemistry and finally, in tissues, organs and organisms, the brain being the most complex global expression of chemical non-linearities so far known (King 2008, 2012), forming "the three-pound universe" (Hooper and Teresi).
The hierarchical process leading to molecular complexity involves all the forces in sequence. Quarks are bound by colour force gluons into composite particles, such as the proton p+ and neutron n. These then interact by the strong force, via the nucleosynthesis pathway, to form the elementary nuclei. The nucleosynthesis pathway generates over a hundred atomic nuclei from the already composite proton and neutron. Parity between protons and neutrons is mediated by weak decay and is slightly broken at lowest energies to balance filling nuclear quantum levels with increasing electromagnetic repulsion of the positive protons, fig 5(b). Nucleosynthesis is a complex process catalytically moderated by several of the isotopes of lighter elements such as carbon and oxygen. Subsequently the weaker electromagnetic force interacts, firstly by formation of atoms through aggregation of electrons around nuclei and then by secondary interaction of complete atoms to form molecules. Molecular bonding is a non-linear quantum interaction, which is never fully resolved and thus perpetuates in a sequence of stages through successive strong and weak bonding interactions, making possible the complex tertiary structures of biomolecules.

Fig 4b: Nucleosynthesis pathways involve multple processes. H and He are made in the big bang. The central elements of life from carbon on are made in the nucleosynthesis processes of small and large stars, leading to C12 and O16 as stable intermediates. Heavier elements are made both by the slow s-processes of large stars and the rapid r-processes of supernovae, where there is an intense flux of neutrons driving the process up to heavier less sable nuclei. Fe is the most stable atomic nucleus with energy able to be released both by fusion and by fission. Occurrence of gold, lead, platinum, uranium and other heavy elements was discovered by Hubble after a gamma ray burst attributed to colliding neutron stars (Wayman 2013). More recently a colliding pair of neutron stars was detected by both gravitational waves and an explosion giving off a spectrum of radiation from radio to x-rays, including a short gamma ray burst giving an understanding of where these come from. The spectography indicates that the collision generated 10 times Earth's mass in gold and that such processes could account for around 47% of the heavy elements in the universe. A nearby binary neutron-star merger about 1,000 light-years away from our Pre-solar Nebula is estimated to have given birth to 0.3% of the Earth's heaviest elements, including igold, platinum and uranium, and about an eyelsh of iodine in each of us, approximately 80 million years before the formation of our Solar System (Bartos & Szabolcs 2019). The dwarf galaxy Reticulum II has also been discovered to have seven stars with heavy elements, like europium, gadolinium and neodymium, in just the proportions produced by the r-process in which massess of neutrons decay into heavy nuclei by emitting electrons (Ji et al. 2016). Europium has an emission spetrum in the visible which makes it particularly good to observe as its lines are not absorbed by Earth's atmosphere liek those of elements with lines in the ultra-violet. The rareness of such enriched dwarf galaxies, eliminating core-collapse supernovae as the source suggests a single event such as a collision of two neutron stars, or a rare type of stellar explosion that spews jets of material. Collapsars, old large stars than can collapse to black holes have also been predicted in a theoretical study to emit a spectrum of heavy metals by the r-process, which has been estimated to produce 80% of the heavy metals on Earth (Siegel et al. 2019). More recently, the picture has become more complicated, with both collapsing neutron stars and supernovas contributing, and gold's origins remain somewhat of an enigma between the two (Croswell 2021).
Generation of the chemical nuclei requires a cosmic cycle through the supernova explosion of a short-lived hot star, generation of heavier elements like gold possibly involving the collapse of twin neutron stars after supernova formation (Rosswog) and/or Collapsars (Siegel et al. 2019). In the second phase, these elements are drawn into a lower energy long-lived sun-like star, the lighter elements associated with terrestrial biology occur in relatively high abundance as a result of nucelosynthesis dynamics, fig 5(a), and can become concentrated on mid-range planets. The final re-entry of the forces occurs through irradiation of molecular systems from photons emitted by stellar thermal radiation, representing the final re-interaction of the residual lower energy electromagnetic bosons with their fermionic counterparts in the electromagnetic orbitals of molecules. The typical coupling of the 5000oC surface temperature of sun-like stars provides photonic energy suitable for energizing weak-bonded molecular structures, without destroying them. A pivotal environment in which this final negentropic low-energy re-entry occurs in abundance are the surfaces of rocky planets in the temperature belt where water is liquid. The variety of planetary systems so-far discovered demonstrates the capacity of the universe to explore through chatoic non-linearities in gravitational orbits, a diverse array of planetary surfaces, ensuring the phase space of potential molecular environments is well explored on a cosmic scale (fig 8).
 Fig 5: (a) Cosmic abundances of the bioelements. (b) The neutron excess of the stable nuclei reflects the interaction between the strong and electromagnetic forces via the weak force
Fig 5: (a) Cosmic abundances of the bioelements. (b) The neutron excess of the stable nuclei reflects the interaction between the strong and electromagnetic forces via the weak forceThe Anthropic cosmological principle introduces the existence of observers as a boundary condition, effectively imposing the existence of life as a cosmological constraint. It asserts that fundamental properties of the universe may have been selected by the fact that only with such constraints on the laws of nature would there be a (complex biological) observer to witness the universe and examine its laws (Barrow and Tipler). Forms of many-universes or many-histories cosmology likewise allow for a spectrum of possible universes, only some of which would have laws of nature which would permit the complex interactive states we associate with living systems. Some cosmologies suggest selection principles may regenerate the universe as a whole, and predispose it to the complexity we find evident (Smolin 1997).
A key approach which seeks to define the laws of nature uniquely derives from super-symmetric string theories. In supersymmetry, each half-integer spin matter-forming fermion (e.g. electron, proton, neutrino, fig 4) is matched by a force/radiation-generating integer spin boson (e.g. Higgs, photon, Zo, gluon, graviton, fig 4), although evidence for this at the LHC has been evaporating since the discovery of the Higgs boson (Slezak 2012) and other theories suggest a non-symmetric contibution based on the exceptional simple group E8 (Lissi 2007). In string theories point particles become resonant loops, strings or membranes in higher dimensional space as distance shrinks, avoiding the infinite singularity of point particles. Consistent super-'brane' theories (Green 1985, 1986, Mukerjee 1996, Duff 1998) require a large number of dimensions, between 10 and 26 in which all but four dimensions (space-time) curl up on microscopic scales. Despite millions of possible compactifications, none has so far been defined which matches our particles and forces. Regardless of the fine details of the ultimate theory resolving the origins of the universe in unification, the formof the forces as we know them is consistently described as a conseuqence of symmetry-breaking.


Fig 6: Left: The Orion nebula the closest star-forming region to Earth with stars massive enough to heat up the surrounding gas at 1500 light years, contains newly forming stellar planetary systems, including the propylid (centre rectangle) with a dark 'planetary' disc (top and centre right) (Hubble telescope). Inset lower-left: Some of these newly forming stars are also surrounded by clouds of HCN and HCHO (Buhl). Right: A series of protoplanetary discs in Orion pictured by Hubble set in silouette by illumination from the brightest star in the cluster, Theta 1 Orionis C (spacetelescope.org/news/heic0917/). Right Two young stars with emerging planetary systems imaged by ALMA, the Atacama Large Millimeter/submillimeter Array. Far right: Putative young still-glowing planets observed by the James Webb telescope (arXiv:2310.01231)
THE ABUNDANTLY FECUND UNIVERSE
As time passes, more and more evidence is accumulating that, the universe and its galactic gas clouds are abundant in organic chemicals, from the simplest molecules to sugars, amino acids and nucleic acid bases. Since Fred Hoyle coined the term "wooden universe" based on infra-red spectral data indicative of carbohydrate emission, there has been an awareness of the potential of galactic gas clouds to be cosmically abundant sources of prebiotic molecules.
Radio-telescope data as early as 1974 (Buhl) demonstrated clouds of multiple-bonded HC≡N and H2C=O spanning the region in the Orion nebula where several new stars are forming, fig 6. More recently, surveys from Hershel have produced high resolution maps of the distributions of HCN, and ionized H2CO and NH3 in both the Perseus and Serpens molecular clouds (Storm et al. 2014). HCN is ubiquitous in the universe and had by the 1980s also been detected in other galaxies (Henkel et al. 1988). A hint of the diverse small molecules found in interstellar molecular clouds can be found in Helmich & van Dishoeck (1997).

Fig 6b: The milky way is permeated with "yellow balls" which are clusters of newborn stars (Wolf-Chase et al. 2021).
These molecules are key precursors of complex polymerization pathways discussed below. Glycine has also been found in interstellar gas and adenine is an abundant product in simulations of collapsing interstellar gas clouds containing a dozen elements including hydrogen, carbon, oxygen and nitrogen (Chakrabadi 2000). The first chiral molecule propylene oxide, CH3CHCH2O has also been found around the embedded, massive protostellar clusters in the Sagittarius B2 star-forming region (McGuire et al. 2016). The nebula sits near the center of the galaxy and has historically been a rich hunting ground for interstellar molecules. The cloud was loaded with propylene oxide. The stockpile has a mass equal to about 80 percent of Earth's mass. The observations don't reveal whether the cloud has a preference for one handedness over another.
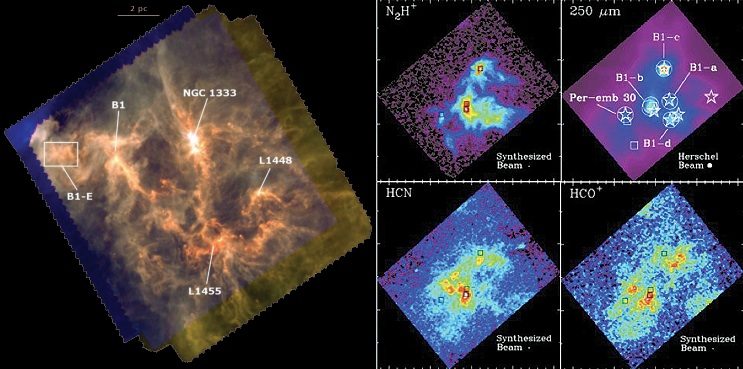

Fig 6b: (left) The Perseus molecular cloud (Sadavoy et al. 2010) with distributions of HCN and ionized H2CO and NH3 (Storm et al. 2014).
(right) Young star AB Aurigae 520 light years from Earth shows a 'twist' marking the spot where a new planet is being formed.
Glycolaldehyde has been detected by Jan Hollis (2000) in a cloud of gas and dust 2 light years across of a type from which new stars are formed. He notes "Interstellar clouds are spread throughout the galaxy and you often find the same molecule in many different clouds. Since these organic molecules are so widespread, it may mean that pre-biotic chemical evolution is an ongoing process." Glycoaldehyde can combine with other carbohydrate molecules to produce ribose. Iso-propyl cyanide has been detected in a star-forming cloud 27,000 light-years from Earth.
 Fig 7: Left: The cloud from which glycoaldehyde has been detected. Right Droplets made by harsh radiation under interstellar conditions (Dworkin et. al.)
Fig 7: Left: The cloud from which glycoaldehyde has been detected. Right Droplets made by harsh radiation under interstellar conditions (Dworkin et. al.)There is now evidence for both water and carbonaceous pre-organics on a cosmological basis. Solar system and interstellar dust grains have been found to contain both water, generated through the bombardment of activated hydrogen from the solar wind onto the silicate mineral oxygen atoms in the dust grains, and a major component of between 40% and 80% by searched area of carbon-containing molecules. These can be found still falling into the snows of Antarctica. This means that the dust in the solar system is basically composed of the elements of organic life as we know it. The dust grains both show evidence of formation in the extreme cold of the outer Oort cloud from deuterium-hydrogen ratios of 25-30%, and evidence of mixing between granules formed in the hotter inner solar system then flung further out, as well as cometary material falling inwards and asteroids further fragmenting and mixing the ensuing dust cloud. This leads to an understanding that the molecules of life originate from the interstellar dust left behind by planetary accretion which then take root on a rocky planet where liquid water can exist.
Volcanic basalt rocks on Baffin Island in the Canadian arctic contain tiny glassy inclusions that appear to have been preserved deep in the mantle for about 4.5 billion years, making them almost as old as the planet itself. These early rocks contain a deuterium ratio nearly 22 per cent less than in seawater today. Instead, the ratio suggests that the water must have originated in the dust cloud from which the sun and planets originally condensed implying that rather then hot and dry until bombarded with water the early Earth had water present from the outset (doi:10.1038/nature.2015.18779).
Astronomers have also discovered the largest and oldest mass of water ever detected in the universe - a gigantic cloud hundreds of light years across, 12-billion-years-old , harboring 140 trillion times more water than all of Earth's oceans combined, surrounding the supermassive black hole of a quasar located 12 billion light-years from Earth. Hence this is water that was present only some 1.6 billion years after the beginning of the universe.
The dust grains floating through the Solar System contain tiny pockets of water, generated by the charged solar wind (Bradley et al. 2014). Combined with previous findings of organic compounds in interplanetary dust, the results suggest that these grains contain the basic ingredients needed for life. As similar dust grains are thought to be found in solar systems all over the universe, this provides a basis for the existence of life across the cosmos . The high D/H ratios, the high organic matter content, and the associated minerals favor an origin from the cold regions of the protoplanetary disk, while the crystalline minerals embedded in the micrometeorite organic matter suggest a solar system rather than interstellar origin (Duprat et al. 2010).
Kwok & Zhang (2011) observing interstellar infra-red signals report that the data are most consistent with the carriers being amorphous organic solids with a mixed aromatic-aliphatic structure. This structure is similar to that of the organic materials found in meteorites, as would be expected if the Solar System had inherited these organic materials from interstellar sources.
In 2012 Glycoaldehyde was found in a gas cloud surrounding the 10,000 year old sun-like star IRAS 16293-2422. By 2013 the list included as well as alcohols and glycoaldehyde, aminoacetonitrile (an amino-acid precursor in 2008 in Sagittarius B2, glycine (amino acid from the NASA Stardust mission on the surface of the comet 81P/Wild 2 in 2009), ethanimine (a nucleic acid precursor in 2013 in Sagittarius B2), benzene, and buckyballs spotted in the environment of an ageing star by NASA's Spitzer Space Telescope in 2010, bringing the tally of complex organics to 180. Earth simulations of the harsh conditions of space have also shown that amino acids and oligopepdies can be formed. In 2012, methoxy radicals were found in interstellar space, leading researchers to discover that quantum tunnelling at these low temperatures assists reaction processes which could not occur at higher temperatures. Comets have been shown to have primordial solar system organics. Primitive polymerizations have been shown to occur during cometary bombardment of the rocky planetary surface possibly explaining how life appeared rapidly on the early earth after a period of heavy cometary and meteorite bombardment (Mueck 2013).
A team led by David Deamer and co-workers has also formed complex organic molecules under the harsh conditions of outer space. The main ingredients of interstellar ices are simple chemicals frozen together. Mostly water, some ammonia, carbon monoxide, carbon dioxide and methanol. The team froze a mixture of these chemicals into a thin solid ice at temperatures close to absolute zero (-441oF/ -263oC) under extreme vacuum and exposed this to harsh ultraviolet radiation that mimics the radiation in space produced by neighboring stars. Instead of finding a handful of molecules only slightly more complicated than the starting compounds, hundreds of new compounds were produced in every mixed ice studied. The types of compounds produced are strikingly similar to many infalling meteorites and interplanetary dust particles. Thus much of the organic material found on the Earth in its earliest years probably had an interstellar heritage." (Dworkin et. al. 2001).
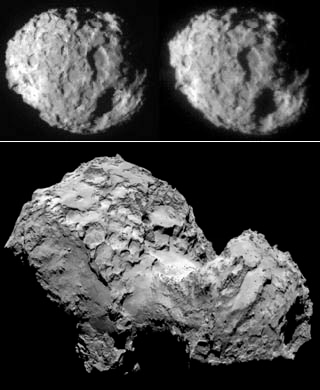
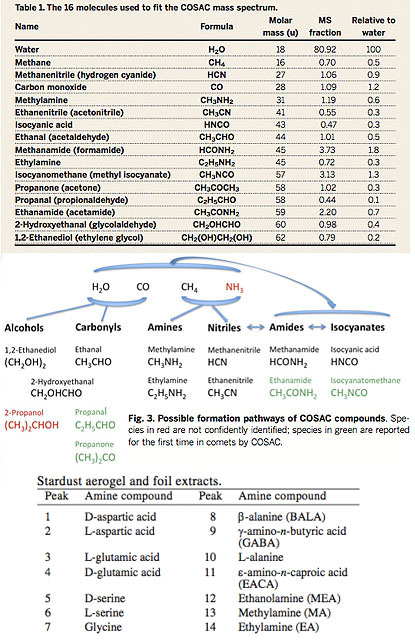 Fig 7a: Left: A stereo view of Wild-2 (above) and Churyumov-Gerasimenko (below). Right: Organics detected in the COSAC experiment on Philae (above). The team saw no signs of amino acids, but they did show up in samples from NASA's Stardust mission, (below) which returned material from the tail of comet Wild 2 to Earth.
Fig 7a: Left: A stereo view of Wild-2 (above) and Churyumov-Gerasimenko (below). Right: Organics detected in the COSAC experiment on Philae (above). The team saw no signs of amino acids, but they did show up in samples from NASA's Stardust mission, (below) which returned material from the tail of comet Wild 2 to Earth.
Cometary impacts are believed to have coated the Earth in a rich endowment of organics from the earliest stages of solar system evolution when impact rates were high. Diverse organic molecules (fig 7c) have also been found in comet 67P/Churyumov-Gerasimenko accessed by Rosetta and the Philae lander (Wright et al. 2015, Goessman et al. 2015) representing some of the most pristine original material from the formation of the solar system. No amino acids were deteced, but the NASA Stardust mission detected several amino acids (Glavin et al. 2008) from the tail of comet Wild 2 (fig 7c). The δ13C value for glycine of +29 +/- 6% strongly suggests an extraterrestrial origin (Elsila et al. 2009). The Rosetta probe has found a mixture of "rotten eggs, cat urine and bitter almonds" - hydrogen sulphide, ammonia and hydrogen cyanide - indicating comets are a rich source of key prebiotic organics. The probe has since definitively seen glycine in the gas cloud surrounding the comet as well as a specturm of other organics including methylamine and ethylamine. The probe also picked up the scent of phosphorus, essential to nucleic acids. Previously it had found alcohols, sugars and oxygen compounds, which are also needed for life and cellular structure. With the addition of glycine and phosphorous, all the major types of prebiotics have been found on the comet (Altwegg et al. 2016).
 In 2020 ALMA (Atacama Large Millimeter/Submillimeter Array), has allowed a detailed look into the star-forming region AFGL 5142, enabling astronomers to pinpoint where phosphorus-bearing molecules, like phosphorus monoxide, form. Flows of gas from young massive stars open up bidirectional cavities in the interstellar clouds surrounding the outflow axes. Molecules containing phosphorus form on the cavity walls (right), through the combined action of shocks and radiation from the infant star. Phosphorus monoxide is the most abundant phosphorus-bearing molecule in the cavity walls. The search then moved back to comet Churyumov-Gerasimenko and ROSINA (Rosetta Orbiter Spectrometer for Ion and Neutral Analysis . If the cavity walls collapse to form a star, particularly a less-massive one like the Sun, phosphorus monoxide can freeze out and get trapped in the icy dust grains that remain around the new star to form pebbles, rocks and ultimately comets, which become transporters of phosphorus monoxide. After the ALMA observations suggested that phosphorus monoxide would be a very likely phosphorus molecule, the scientists went back to their data and found evidence of this molecule on the comet (Rivilla et al. 2020).
In 2020 ALMA (Atacama Large Millimeter/Submillimeter Array), has allowed a detailed look into the star-forming region AFGL 5142, enabling astronomers to pinpoint where phosphorus-bearing molecules, like phosphorus monoxide, form. Flows of gas from young massive stars open up bidirectional cavities in the interstellar clouds surrounding the outflow axes. Molecules containing phosphorus form on the cavity walls (right), through the combined action of shocks and radiation from the infant star. Phosphorus monoxide is the most abundant phosphorus-bearing molecule in the cavity walls. The search then moved back to comet Churyumov-Gerasimenko and ROSINA (Rosetta Orbiter Spectrometer for Ion and Neutral Analysis . If the cavity walls collapse to form a star, particularly a less-massive one like the Sun, phosphorus monoxide can freeze out and get trapped in the icy dust grains that remain around the new star to form pebbles, rocks and ultimately comets, which become transporters of phosphorus monoxide. After the ALMA observations suggested that phosphorus monoxide would be a very likely phosphorus molecule, the scientists went back to their data and found evidence of this molecule on the comet (Rivilla et al. 2020).
The capacity of complex organic molecules generated in space to enter Earth's atmosphere intact has also been confirmed. Jeffrey Bada has found evidence that 'mother lodes' of buckyballs, football-shaped molecules made up of carbon atoms, have fallen intact to Earth from outside the Solar System from Sudbury, Ontario, where a meteoroid the size of Mount Everest crashed 2 billion years ago. They were loaded with helium, an element rare on Earth, but abundant in inter-stellar space. The single impact site contained about 1 million tons of extra-terrestrial buckyballs. If complex buckyballs could fall on earth without burning up so could complex organic molecules (Cohen 1996).
It has also been proposed that Earth may have been subject to lithopanspermia when the star cluster forming the sun and neighbouring stars first formed (Belbruno et al. 2012). In this scenario, weak transfer due to collision fragments escaping one star system and wandering into another could allow not only complex organics but primitive life forms to be transferred. This extends beyond the transfer of meteorite material between Solar System planets, which could likewise mean that life could have been transferred from Mars to Earth by a similar process. Mars may have been hospitable to life before Earth was and had a more mixed wet and dry surface conducive to biogenesis, with borates conducive to cyclic sugar formations and oxidized molybdenum capable of early photosynthesis (Redfern 2013). Meteorites of Martian origin have more recently come to the Earth. From magnetic studies, some reached temperatures no higher than 40oC allowing transfer of living systems (http://all-geo.org/metageologist/2012/11/earth-moon-and-mars/) The advent of the interstellar remnant Oumuamua has led to fresh estimates of galactic mixing through transfer of small cometary objects between star systems (Ginsburg, Lingam & Loeb 2018). Panspermia could explain why life began so rapidly on Earth but the cosmological basis of life's origin remains the central issue to be resolved.
 Fig 7b: (Top-left) Relative abundances of amino acids in the Murchison chondrite and Spark syntheses (Kvenvolden et al. 1972). (Top-right) Portrait of molecular diversity in the Murchison meteorite (Schmitt-Kopplin et al. 2009). (Lower-left) Coherent lithopanspermia time scales (Belbruno et al. 2012). (Lower-centre) Murchison meteorite with mineral inclusions in enlarged inset. (Lower-right) Candidate interstellar molecule (Kwok & Zhang (2011). Click to enlarge.
Fig 7b: (Top-left) Relative abundances of amino acids in the Murchison chondrite and Spark syntheses (Kvenvolden et al. 1972). (Top-right) Portrait of molecular diversity in the Murchison meteorite (Schmitt-Kopplin et al. 2009). (Lower-left) Coherent lithopanspermia time scales (Belbruno et al. 2012). (Lower-centre) Murchison meteorite with mineral inclusions in enlarged inset. (Lower-right) Candidate interstellar molecule (Kwok & Zhang (2011). Click to enlarge.
Chondrites are stony (non-metallic) meteorites that have not been modified due to melting or differentiation of the parent body.They are formed when various types of dust and small grains that were present in the early solar system accreted to form primitive asteroids. They are the most common type of meteorite that falls to Earth with estimates for the proportion of the total fall that they represent varying between 85.7% and 86.2%. One of their characteristics is the presence of chondrules, which are round grains formed by distinct minerals, such as olivine that normally constitute between 20% and 80% of a chondrite by volume.
Carbonaceous chondrites (also known as C-type chondrites) make up less than 5% of the chondrites that fall on Earth. They are thought to have been formed the farthest from the sun of any of the chondrites as they have the highest proportion of volatile compounds. Another of their main characteristics is the presence of water or of minerals that have been altered by the presence of water. Carbonaceous chondrites contain more than 600 organic compounds that were synthesised in distinct places and at distinct times. These organic compounds include: hydrocarbons, carboxylic acids, alcohols, ketones, aldehydes, amines, amides, sulfonic acids, phosphonic acids, amino acids and nitrogenous bases. The first fraction (not soluble in chloroform or methanol) appears to originate from interstellar space and the compounds belonging to the other fractions (chloroform soluble hydrocarbons and menthanol soluble including amino acids) derive from a planetoid (small planetary aggregations including asteroids and Kuiper Belt objects). It has been proposed that the amino acids were synthesized close to the surface of a planetoid by the radiolysis (dissociation of molecules caused by radiation) of hydrocarbons and ammonium carbonate in the presence of liquid water. In addition, the hydrocarbons could have formed deep within a planetoid by a process similar to the Fischer-Tropsch process. and may have played a part in the process resulting in life's origin on Earth. caused the origin of life on Earth.
The Murchison meteorite is a CM2 chondrite characterised by the mineral presence of phylosilicates and olivine and contains common amino acids such as glycine, alanine and glutamic acid as well as other less common ones such as isovaline and pseudo-leucine. Two meteorites that were collected in Antarctica in 1992 and 1995 were found to be abundant in amino acids, which are present at concentrations of 180 and 249 ppm (carbonaceous chondrites normally contain concentrations of 15 ppm or less). This could indicate that organic material is more abundant in the Solar System than was previously believed, and it reinforces the idea that the organic compounds present in the primordial soup could have had an extraterrestrial origin.
Along with amino-acids, all the nucleotide bases A, U, G, and C have been detected in carbonaceous chondrites (Hua et. al. 1986), such as the Murchison meteorite. These also contain amphophilic membrane forming products (Deamer and Pashley 1989). Over 15 amino acids have been identified in the Murchison meteorite (fig 7b), a carbonaceous chondrite containing primitive material from the Solar System's origin chemically altered by water during time on asteriodal bodies, before falling to Earth. The amino acids found in the Murchison meteorite have also been synthesized in laboratory experiments by the action of electric discharge on a mixture of methane, nitrogen, and water with traces of ammonia (Kvenvolden et al. 1972). A complex mixture of alkanes was isolated as well, similar to that found in the Miller-Urey experiment. Alanine has been found to have excess of the L-enantiomer and in 1997, L-excesses were also found in a non-protein amino acid, isovaline, suggesting an extraterrestrial source for molecular asymmetry in the Solar System. A team demonstrated in 2005 that this homochirality could have been triggered or catalyzed, by the action of a left-handed amino acid such as proline (Cordova et al. 2005). Measured purine and pyrimidine compounds, including guanine, cytosine, adenine, thymine and uracil and others such as xanthine are indigenous components of the Murchison meteorite. Carbon isotope ratios for uracil and xanthine of δ13C = +44.5% and +37.7%, respectively, indicate a non-terrestrial origin for these compounds (Martins et al. 2008). These results demonstrate that many organic compounds which are components of life on Earth, were already present in the early Solar System and may have played a key role in life's origin. The full complexity of the tens of thousands of organic molecules and likely millions of diverse structures has been assayed by (Schmitt-Kopplin et al. 2009). They comment that this molecular complexity, which provides hints on heteroatoms chronological assembly, suggests that the extraterrestrial chemodiversity is high compared to terrestrial relevant biological- and biogeochemical-driven chemical space.
Ribose has been found in three carbonaceous chondrites, including the Murchison (Furukawa et al. 2020). The ribose in the Murchison of 45 ppb also has C13 levels 43% higher than terrestrial confirming an extraterrestrial origin. The δ13C values of ribose and xylose in NWA 801 and ribose and arabinose in Murchison are comparable to those of sugar acids and sugar alcohols detected previously (i.e., +5 to +82% in GRA 95229 and Murchison meteorites) and those of α-amino acids in many carbonaceous chondrites, which typically range from +3 to +44% . This is very strong evidence that NWA 801 and Murchison contain extraterrestrial pentoses). A variety of extra-terrestrial nucleotide bases have also been found, including all biological purine and pyrimidine bases (Callahan et al. 201, Oba et al. 2022).
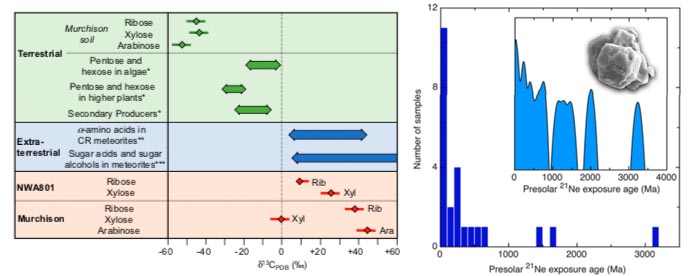
Fig 7c: Ribose and xylose left and silicon carbide crystals right.
Just how long and complex the history of the Murchison Meteorite is can be gleaned from the discovery of silicon carbide crystals in it dating from as far back as 7500 billion years ago, nearly twice the age of the sun and solar system (Heck et al. 2020) . When stars die, particles formed within them are flung out into space. These "pre-solar grains" then get incorporated into new stars, planets, moons and meteorites. Their age can be calculated based on cumulative radioactive bombardment as a "continuous rain of change" . The analysis process starts with crushing fragments of the meteorite down into a powder. Once all the pieces are segregated, it's a kind of pungent paste, smelling like rotten peanut butter. This paste was then dissolved in acid, leaving only the stardust. Their ages, based on cosmogenic Ne-21, range from 3.9 ± 1.6 million years to ~3 ± 2 billion years before the start of the Solar System ~4.6 billion years ago. A majority of the grains have interstellar lifetimes of <300 Ma, which is shorter than theoretical estimates for large grains. These grains condensed in outflows of asymptotic giant branch stars <4.9 Bya ago that possibly formed during an episode of enhanced star formation ~7 Bya ago. A minority of the grains have ages >1 Ga. Longer lifetimes are expected for large grains. At least 12 of the analysed grains were parts of aggregates in the interstellar medium: The large difference in nuclear recoil loss of cosmic ray spallation products 3He and 21Ne indicate that the irradiated objects in the interstellar medium were up to 30 times larger than the analysed grains. Furthermore, the majority of the grains acquired the bulk of their cosmogenic nuclides in the interstellar medium and not by exposure to an enhanced particle flux of the early active sun.
Large L-enantiomeric excesses (Lee ~ 43-59%) of aspartic and glutamic acids were found in the Tagish Lake C2-type carbonaceous chondrite, whereas alanine was found to be nearly racemic (D ~ L). Carbon isotope measurements of D- and L-aspartic acid and D- and L-alanine (fig 7d) indicate that the L-aspartic acid enrichment is indigenous to the meteorite (Glavin et al. 2012). These results can be explained by differences in the solid-solution phase behavior of aspartic acid, which can form conglomerate enantiopure solids during crystallization, and alanine, which can only form racemic crystals. The researchers propose that, in the solar system’s early days, heating as a result of radioactivity could have melted ice trapped deep inside asteroids. Liquid water then dissolved already present amino acids, which crystallised into mostly left-handed groupings. Amplification of a small initial L-enantiomer excess during aqueous alteration on the meteorite parent body could have led to the large L-enrichments observed for aspartic acid and other conglomerate amino acids. The detection of nonterrestrial L-proteinogenic amino acid excesses in the Tagish Lake meteorite provides support for the hypothesis that significant enantiomeric enrichments for some amino acids could form by abiotic processes prior to the emergence of life. An excess of L-amino-acids of 2-18% extends to virtually every meteorite example tested (Chown 2010).



Fig 7d: Left: L-enantiomer excesses and carbon 13 ratios in the Tagish Lake chondrite. Centre-right: Hemolithin an extraterrestrial protein. Right: Meteoritic organometallic compounds compared to active hydrogenase sites. (a) [FeII(CN)5(CO)]3− (boxed) and active-site structure of [NiFe]-hydrogenase from Desulfovibrio gigas (1FRV). (b) [FeII(CN)4(CO)2]2− (boxed, cis form shown) and active-site structure of [FeFe]-hydrogenase from Clostridium pasteurianum (3C8Y). Regions that are shaded blue indicate structural similarity. A bridging ligand between metals is not shown for clarity.
Amino acid polymers previously observed in the Acfer 086 and Allende meteorites have been further characterized in Acfer 086 via high precision MALDI mass spectrometry to reveal a principal unified structure of Hemolithin: a Meteoritic Protein of molecular weight 2320 Daltons that involves chains of glycine and hydroxy-glycine residues terminated by iron atoms, with additional oxygen and lithium atoms (McGeoch, Dikler & McGeoch 2020).
Smith et al. (2019) have identified several complex cyanide compounds in a set of CM chondrite meteorites (fig 7d right). These extraterrestrial organometallic compounds are a source of free cyanide and also bear a striking similarity to portions of the active sites of hydrogeneses (enzymes that provide energy to bacteria and archaea by breaking down hydrogen gas), which suggests that these compounds may have played an important role during the origin and early evolution of life on Earth.
Scenarios for the early Earth's atmosphere suggest a predominantly CO2 atmosphere with little or no free O2 and much lower levels of N2 than at present. Thus incoming carbonaceous material from the primordial solar system rich in HCNO molecules as we see in the Murchison and Tagish Lake chondrites would not have been oxidized. Furthermore in the Hadean atmosphere, lasting through to the earliest evidence of life on Earth around 3.8-3.5 billion years ago, fixation of N2 from the atmosphere by lightning creating reactive NO would have continued until the CO2 levels dropped, so that by around the 2.2 billion year mark, the production of NO declined, leading to the return of nitrogen to the atmosphere. It is thus believed that most nitrogen was sequestered in the Earth's crust and oceans during the Hadean and the nitrogen dominated atmosphere arose after the fall in atmospheric CO2 to levels also promoting the accumulation of N2 in the atmosphere (Navarro-Gonzalez et al. 2001).
The extreme variety of conditions on our own planets and between the moons of Jupiter and Saturn are only a foretaste of the bizarre variety of planets detected around other neighbouring stars. This extreme variety is consistent with the non-linear nature of gravity under inverse square law attraction in four-dimensional space-time and the resulting capacity of the universe to explore its own space of possibilities through chaotic dynamical interaction.
There are of course multiple factors contributing to these differences. The major planets have a graduated sequence of temperatures falling precipitously from the 428oC of Mercury (with the dark side being as low as -179oC due to its slow rotation), Venus has an even higher temperature at 471oC due to runaway global heating, Earth is at an average of 15oC also now subject to global heating, Mars at -28oC, Jupiter at -108oC, Saturn at -138oC, Uranus -195oC, Neptune -204oC and Pluto -233oC. This means that the inner planets are rocky with only a smattering of organic compounds, while the gas giants from Jupiter on have thick atmospheres with copious organic carbon-containing molecules. Research has also found that these fall into two distinct classes with the rocky planets asteroids and non-carbonaceous meteorites having distinct isotope ratios from the outer planets and carbonaceous chondrites (Warren 2011), indicating the these two populations arose from separate accretion bands in the nascent solar system (Brasser & Mojzsis 2020), as illustrated in Orion nebula star formations in fig 6.
Specific events have also shaped their differences. Venus has undergone a catastrophic change into a state of runaway global heating and may have previously had water and could possibly have supported life for 3 billion years(Way 2019). Earth's moon suggests an early collision between Earth and a Mars-sized object also shaping its history. Mars lost its magnetosphere 4 billion years ago, so the solar wind interacts directly with the Martian ionosphere, lowering the atmospheric density by stripping away atoms from the outer layer.
However these factors alone are not enough to explain the differences between the planets, which are also repeated in the major moons of Jupiter and Saturn where all the moons have coalesced from smaller particles from pebble size up (Shibaike et al. 2019) under similar conditions and yet display diverse atmospheres and compositions.
Both Titan and Enceladus have been singled out in terms of their potentials to supports forms of life. Titan has a thick atmosphere and is thought to be a prebiotic environment rich in complex organic compounds, but its surface is in a deep freeze at -179oC. However, Titan seems to contain a global ocean beneath its ice shell, and within this ocean, conditions are potentially suitable for microbial life. Although Enceladus is small in size and shrouded in a thick shell of ice, it appears to be a habitable world. It has a source of energy from friction created by its orbit around Saturn, organic compounds that are building blocks for life and a liquid water ocean underneath that ice, which drives over 90 geysers that spew plumes of salty water vapor, organic compounds and ice particles from the underground ocean into the air.

Fig 8: Above, the solar system planets Mercury, Venus, Earth, Mars, Juliter, Saturn, Uranus, Neptune and Pluto. Lower left, the four Galilean moons of Jupiter
Europa, Io, Callisto and Ganymede. Lower right, six principal moons of Saturn Titan, Rhea, Lapetus, Tethys, Dionne and Enceladus with its blue icy pleated surface.
Given this chaotic exploration of planetary conditions, the many planets already discovered have even more diverse conditions than those of our own solar system. Because of the techniques used to locate them, such as disturbances to stellar position, some of these have inevitably turned out to be Jupiter-like gas giants, but as techniques of planet location have become more acute it has become established that some planets are inevitably in the "goldilocks" zone with liquid water where life as we know it could exist. Kepler, which detects luminosity variations, has already found 2740 planets, 461 of which are Earth-sized, 10 of which are in the habitable zone of liquid water (Palmer 2013). One with a size and orbit similar to Earths's has been recently found orbiting a sun-like star whose atmosphere might be similar enough to ours to support Earthly plant life (Clery 2015). The number of planets in our galaxy is now deduced to be in the billions and planets in the universe to be in the trillions, so the notion that we are alone in the universe having a planet that can support life is no longer a realistic proposition.


Fig 8b: Left the habitable zone of liquid water. Centre: Testing the Titus-Bode law (right) on exoplanets.
New calculations in a 2015 study (Boviard et al.) indicate that billions of the Milky Way's stars have one to three planets in the habitable zone, meaning that they potentially have liquid water as well. The Titius-Bode law, created around 1770, predicts how planets in a solar system will be spaced out. The researchers applied the law to the 1,000 exoplanets (and 3,000 possible exoplanets) found by NASA's Kepler satellite. They looked at 151 planetary systems - ones where Kepler had detected between three and six planets - and found that the Titius-Bode law fits well with the way 124 of them were spaced out. In the planetary systems where ratios were off, they were able to estimate where "missing" planets might be. Once those planets were added, all 151 systems showed one to three planets in their habitable zone. The researchers believe this indicates that most systems do have planets orbiting at the proper distance to hold liquid water. It has been calculated that giant elliptical galaxies might host 10,000 times as many habitable planets as elliptical galaxies like ours (Dayal et al. 2015).
A new theory of rapid formation of the proto-Earth through the accretion of cosmic dust -- millimetre-sized objects, all coming together, raining down on the growing body and making the planet in one go. This breaks with the traditional theory that it formed by random collisions between larger and larger planetary bodies throughout several tens of millions of years. Not only is this implication of the rapid formation of the Earth interesting for our solar system. It is also interesting to assess how likely it is for planets to form somewhere else in the galaxy. The key came from the most precise measurements of iron isotopes that have so far been published scientifically. By studying the isotopic mixture of the metallic element in different meteorites, the researchers found only one type of meteoritic material with a composition similar to Earth -- the CI chondrites. The dust in this fragile type of meteorite provides our best equivalent to the bulk composition of the solar system itself. It was dust like this combined with gas that was funnelled via a circumstellar accretion disk onto the growing Sun lasting about five million years and our planets were made from material in this disk. The proto-Earth's ferrous core also formed already during this period, removing early accreted iron from the mantle (Schiller, Bizzarro & Siebert 2020).
A Research time-line of planetary discovery.
THE NUB OF BIOCOSMOLOGY
Damer and Deamer (2020) in setting out their "hot spring hypothesis" provide an illustration, fig 8c, containing alternative scenarios for the origin of life. Although their figure seems to comprehensively survey the field, it focuses on volcanism and misses out completely several of the most plausible scenarios, including the lost city vents, fig 24, central to the Lane-Martin-Russel hypothesis, figs 33, 35, which also has pivotal support in genetic analysis of LUCA. These are not volcanic hydrothermal vents as such, but chemical redox gardens, with their cosmologically ubiquitous alkaline olivine reacting with CO2 acidified sea water mentioned nowhere in the paper. This is no fault of Damer and Deamer, who are setting out a volcanic hot spring hypothesis, but it illustrates the hit-and-miss problem facing all current origin of life scenarios and the key precursors and proposed polymerizations leading eventually to a replicating RNA era, around which the origin of life question pivots.

Fig 8c: Alternative scenarios for an origin of life and adaptive pathways from freshwater hydrothermal field pools or saltwater hydrothermal vents to eukaryotes and land plants.
Originally in 1978 I had the idea of making a biochemical version of the LHC and freely adjusting the input parameters until the complexity hit a chain reaction. The trouble with this idea is that it is a single reactor dealing at all times with one set of initial conditions. Likewise the primitive earth synthesess, from Milley-Urey below on, have shown some remarkable molecular complexity which has noticeable affinity with the substances found in the Murchison and other carbonaceous chondrites. However until Sutherlands "one-pot" syntheses, fig 21, there appeared to be no plausible routes to pyrimidine nucleotides, again illustrating the fact that critical changes in the reactants can induce synthesis pathways previously unconceived of, without which the origin of life might seem impossible, in this case with presynthesized heterocyclic bases and suggars forming a road block while other intermediates could assemble in one pot to fully formed nucleotides.
What the universe shows us is that the laws of nature invoke symmetry-broken chaotic processes that generate extreme diversity of conditions. Looking at our own planets we see extreme variation of the chemical dynamics. Although there is a chemical graduation, with a bunch of gassy giants full of organics and an inner set of rocky planets with only a smattering of organics, the sheer diversity, repeated on the satellites of the major planets and extrapolated across the galaxy in star-forming regions like the Orion nebula tell us that just about everything that can possibly happen does. Contrast this with primitive lab syntheses and we are, in each case, invoking a single plausible initial set of a few simple organic molecules inducing a polymerisation that might, or might not, be necessary, or sufficient. It is effectively a single dot in parameter space, or at best five or six isolated points.
Likewise, when we consider ball and stick arguments made in a mechanistic misreading of quantum chemistry sometimes as an apology for religious belief in creation, that claim that, even if the moon were made of green cheese, life would remain improbable to the point of impossible because of the number of unlikely combinations of molecules and events that would have to arise in sequence for the whole shebang to get going. This of course makes no sense because molecular quantum computation and autocatalysis can profoundly alter the probabilities, but the key problem for discovering the origin of life on Earth is that none of the existing hypotheses or polymerization pathways invoke an open set in the parameter space of possible origin conditions to make a plausible viability assessment. They are a small scattering of point samples, which, even taken together, do not go anywhere near to a parameter space-filling sample. So, if we are lucky we might hit upon the "sang raal" royal route, but to not find it immediately doesn't cast doubt on the ability of life to emerge, because the bootstrap process is of necessity structurally unstable thermodynamically, or life would end up a lifeless molecular conglomerate, so it is very far from straightforward to simulate.
Topologically, an open set is a subset of a space such that each point in the set has a neighbourhood containing ALL the closer than epsilon variations in the (parameter) space. What the universe is doing is close to a topological exploration by forming an open subset of the possibilities. Orion and other gassy star-forming nebulae set up vast playgrounds where systems of a huge diversity of stellar and solar system types ensue, because the forces of nature, despite being highly structured, are both symmetry-broken and chaotic. Gravity for example, despite being the closest to classical of the forces sports the 3-body problem of chaos due to its inverse square law. Nuclear dynamics are intrinsically chaotic. Chemistry is a fractal manifestation of electronic bonding given the quantum periodicities in the elements which is likewise non-linear in charge interactions. These chaotic processes lead to ergodicity - the ability of the trajectories of cosmological initial conditions to fill virtually every nook and cranny in probability space in an ultimate mixing process. That's why the search for goldilocks planets turns up such a variety of oddball candidates that also makes us wonder how rare our own planet is, despite most small stars having planetary systems of some sort.
The end result is that cosmology is a process where cosmic symmetry-breaking induces an interactive process in which the entire universe is an origin of life experimental reactor, invoking an open set of initial conditions and the inevitable fecundity of life somewhere as the interactive consummation of cosmic symmetry breaking. And somewhere is enough for panspermia to ensue, through carrying materials from one star system to another, as the silicon carbide grains in the Murchison meteorite, fig 7c, demonstrate by their very age. Hence Biocosmology is not just the study of prebiotic chemistry but the interactive catastrophe of the fecund universe.

Fig 8d: Left - Sabbatical creation (6 days c1250-500BC). Centre - Flammarion engraving The discovery process breaches the "raqiya" (c1880AD).
Physical cosmology from quantum fluctuation to the present (c13.7 bya with origin of life on Earth c3.8 bya ago, some third of the universe's lifetime)
.
Given this completely counterintuitive challenge to our notions of existence, why anyone would have the arrogance to introduce an archaic notion like the monotheistic god of moral retribution into the picture defies both science and nature. Yes the sabbatical creation of Genesis 1 is a sublime allegory, but it defies the manifest natural order. Although light is created before the stars, so that we can see, a declarative act seemingly at a stretch consistent with the cosmic background radiation, the plants are impossibly created before the Sun, Moon and stars. The firmament of the heavens is "raqiya" ![]() , a beaten hemispherical bowl, firmament, or vault of heaven, considered by Hebrews as solid and supporting the 'waters' above, thus dividing the waters above and below, into which the stars are fixed. Earth is a flat domain created by bunching the waters under heaven to one place. Night and day happen before the sun is placed in the heavens. The fishes and whales and the birds are created a day before the land animals and long after the plants. And humanity is made "male and female in our likeness" making God in the form of the 'Elohim a conscious anthropomorphic sexual sentient being as we are. The entire personality-based agent manufacturing life from dust, clay or a male rib, breathing life into being, or pronouncing "Let there be light" as a Logos has no ability to capture any of the formative processes we actually find before us. The universe is showing us that its manifestation is far more than a mere intelligently manufactured design, or even a thought in the mind of God, as the allegory of Vishnu dreaming Brahma out of the lotus in his navel, attended all the while by his faithful female partner Lakshmi describes.
, a beaten hemispherical bowl, firmament, or vault of heaven, considered by Hebrews as solid and supporting the 'waters' above, thus dividing the waters above and below, into which the stars are fixed. Earth is a flat domain created by bunching the waters under heaven to one place. Night and day happen before the sun is placed in the heavens. The fishes and whales and the birds are created a day before the land animals and long after the plants. And humanity is made "male and female in our likeness" making God in the form of the 'Elohim a conscious anthropomorphic sexual sentient being as we are. The entire personality-based agent manufacturing life from dust, clay or a male rib, breathing life into being, or pronouncing "Let there be light" as a Logos has no ability to capture any of the formative processes we actually find before us. The universe is showing us that its manifestation is far more than a mere intelligently manufactured design, or even a thought in the mind of God, as the allegory of Vishnu dreaming Brahma out of the lotus in his navel, attended all the while by his faithful female partner Lakshmi describes.
We thus come to the other elephant in the room - the enigma of consciousness - something we associate with mind rather than matter, and extrapolate into spirit and deity as conscious projections. But this isn't just about mind - it's about matter as well! Scientific Philosopher Philip Goff describes it thus. In the standard scientific view, consciousness exists only in the brains of highly evolved organisms. According to panpsychism, in contrast, consciousness pervades the universe and is a fundamental feature of it - that the fundamental constituents of reality -- perhaps photons, electrons and quarks -- have incredibly simple forms of experience, as perhaps manifested through the wave function and quantum entanglement. And the very complex experience of the human or animal brain is somehow derived from the experience of the brain's most basic parts. By "consciousness," we simply mean experience, as in our own notions of pleasure, pain, visual, auditory or even visionary experience.
What philosophers of science have realised is that physical science, for all its richness, is confined to telling us about the behaviour of matter, what it does. Physics tells us, for example, that matter has mass and charge. These properties are completely defined in terms of behaviour, things like attraction, repulsion, resistance to acceleration. Physics tells us absolutely nothing about what philosophers like to call the intrinsic nature of matter: what matter is, in and of itself. The proposal of the panpsychist is to put consciousness in that hole. Consciousness, for the panpsychist, is the intrinsic nature of matter. There's just matter, in this view, nothing supernatural or spiritual. But matter can be described from two perspectives. Physical science describes matter "from the outside," in terms of its behaviour. But matter "from the inside"- i.e., in terms of its intrinsic nature -- is constituted of forms of consciousness. What this offers us is a beautifully simple, elegant way of integrating consciousness into our scientific worldview, of marrying what we know about ourselves from the inside and what science tells us about matter from the outside.
You can thus find each these issues examined in detail in dhushara research pages, from quantum cosmology, through unravelling the evolving tree of life to space, time and consciousness.
QUANTUM CHEMISTRY AS THE NON-LINEAR SCIENCE OF EMERGENT COMPLEXITY
The complex expressions of chemistry particularly in biology are manifest as a final non-linear interactive consequence of cosmological quantum symmetry-breaking. The stability of the nucleus with increasing nuclear mass number and charge permits an unparalleled richness and complexity of quantum bonding structures around the diverse chemical elements. Electron-electron repulsions, spin-obit coupling, delocalized orbitals (Pullman and Pullman 1963) and other effects, perturb the periodicity of orbital properties and lead to the development of higher-order molecular structures. Although quanta obey linear wave amplitude superposition, chemistry inherits an inverse quadtaic non-linearity in the form of the attractive and repulsive charge interactions caused by re-distributing electrons between orbital systems. Such non-linear interaction, combined with Pauli exclusion, is responsible for the diversity of chemical interaction, from the covalent bond to the secondary and tertiary effects manifest in the complex structures of proteins and nucleic acids. The quadratic nature of charge interaction, leads to a situation in polymeric chemistry akin to the Mandelbrot set, (fig 38a) and which is central in making complex molecules (fig 10) and the scale-dependent structures of tissues possible (fig 38b).
 Fig 9: Although all wave functions obey quantum superposition, the non-linear nature of electronic charge distribution and its resulting occupancy energetics, the Pauli exclusion principle and additional electromagnetic effects results in the non-linear energetics of chemical bonding. This non-linear interaction is never fully resolved by any single bonding step and gives rise through subsidiary weak-bonding interactions to the global interactivity of complex biomolecules and cellular organelles.
Fig 9: Although all wave functions obey quantum superposition, the non-linear nature of electronic charge distribution and its resulting occupancy energetics, the Pauli exclusion principle and additional electromagnetic effects results in the non-linear energetics of chemical bonding. This non-linear interaction is never fully resolved by any single bonding step and gives rise through subsidiary weak-bonding interactions to the global interactivity of complex biomolecules and cellular organelles.The source of this non-linear interaction is the foundation of all chemical bonding, the electric inverse square law of charge interaction. Although the state vector of a quantum-mechanical system is a linear combination of base states, exemplified by the formation of linear combinations of s and p wave functions to form the four sp3 hybrid orbitals, the electrostatic charge of the electron causes orbital interaction to have fundamentally non-linear energetics. The total energy is represented by the resonance integral of the Hamiltonian composed with the wave function, divided by the normalizing overlap integral S.
In the case of the one-electron Hydrogen molecule ion, with Saa= Sbb normalized to 1, we have 2 solutions , as indicated:

The capacity of orbitals, including unoccupied orbitals, to cause successive perturbations of bonding energetics results in an interaction bonding sequence, from strong covalent and ionic bond types, through to their residual effects in the variety of weaker H-bonding, polar, hydrophobic, and van der Waals interactions, merging into the average kinetic energies at biological temperatures (Watson et. al. 1988). These are responsible for secondary structures such as the a-helix of proteins and base-pairing and stacking of nucleic acids, and result in the tertiary and quaternary sturctures determining the global form of large biomolecules and the globally-induced active-site effects central to enzyme action.
Fractal and Chaotic Dynamics in Molecular Systems.
By contrast with the periodic crystalline or random amorphous structures of most minerals, the non-periodic scale-dependent primary, scondary and tertiary structures in proteins and RNA that are critical to establishing the richness of their forms and their bio-activity, fig 10. The almost unlimited variety of monomeric primary sequences induce higher-order secondary and tertiary structures through subsequent folding of the polymer. These are possible only because the non-linearity of charge interaction which causes chemical bonding also gives rise to further residual interactions at lower energies which are resolved by cooperative weak bonding. Proteins are powerful catalysts because the global coherence of action arising from cooperative weak bonding makes for very powerful and responsive active sites. Despite being genetically coded, such molecules form fractal structures both in their geometry and their dynamics (Ansari et. al. 1985, Liebovitch et. al. 1987, 1991).
 Fig 10: Global t-RNA and protein [lysozyme] tertiary structures are the result of hierarchy of strong and weaker chemical bonding interactions operating on a non-periodic secondary structure. Both derive their structures in association with water.
Fig 10: Global t-RNA and protein [lysozyme] tertiary structures are the result of hierarchy of strong and weaker chemical bonding interactions operating on a non-periodic secondary structure. Both derive their structures in association with water.Non-equilibrium thermodynamics (Glansdorff and Prigogine 1971) and the associated oscillating chemical systems such as the Beloushov-Zhabotinskii reaction (Epstein et. al. 1983) demonstrate the capacity of auto-catalytic chemical systems, and membrane electrochemistry (Chay & Rinzel 1985), to enter into non-linear dynamics and chaos (Epstein et. al. 1983, Agladze et. al. 1984). Quantum chaos and its suppression is also an emergent issue (Gutzwiller 1992).
In three developments highlighting far-from-equilibrium dissipative dynamics, Kondepudi , Kay & Dixon (2015) have found that the complex motion of beads driven by the applied field, the dipole-dipole interaction between the beads and the hydrodynamic flow of the viscous medium results in a time-evolution of the tree-structure towards states of lower resistance or higher dissipation and thus higher rates of entropy production. Horowitz & England (2017) studied the dynamics of an in silico chemical network with random connectivity in an environment that makes strong thermodynamic forcing available only to rare combinations of chemical concentrations and found that the long-time dynamics of such systems are biased toward states that exhibit a fine-tuned extremization of environmental forcing. Kachman, Owen & England (2017) likewise observed the emergence of an adaptive resonance in a system matched to a drive frequency, and showed that the increased work absorption by these resonant structures was key to their stabilization.
The prebiotic polymerizations leading to the chemical origins of life share an informational paradox in which a small number of simple reactant lead to a large array of complex interacting products with many potential catalytic interactions. The initial conditions are thus insufficient to causally determine the products, except for a few predominant products such as adenine, leading to a huge variety of possible end states with increasing complexity. This allows for a high degree of polymeric variability which can be influenced both by auto-catalytic feedback and stochastic effects.
 Stuart Kauffman and coworkers (Vattay et al. 2015) show that molecules taking part in biochemical processes, from small molecules to proteins, are at a critical transition quantum mechanically. Electronic Hamiltonians of biomolecules are tuned exactly to the critical point of the metal-insulator transition separating the localized insulator phase from the conducting disordered metal phase, providing a partial explanation of why life persists at the edge of chaos - a question at the very heart of evolution as well as biogenesis.
Stuart Kauffman and coworkers (Vattay et al. 2015) show that molecules taking part in biochemical processes, from small molecules to proteins, are at a critical transition quantum mechanically. Electronic Hamiltonians of biomolecules are tuned exactly to the critical point of the metal-insulator transition separating the localized insulator phase from the conducting disordered metal phase, providing a partial explanation of why life persists at the edge of chaos - a question at the very heart of evolution as well as biogenesis.
Fig 10b: (a) HOMO/LUMO (highest occupied/lowest unoccupied) molecular orbitals for Myoglobin calculated with the Extended Huckel method showing self-similarity indicative of fractal dynamics (Vattay et al. 2015). (b) Protein "quakes". Relaxation of excited myoglobin involves four functionally important motions and many equilibrium fluctuations illustrating the basis of fractal energetics in biological molecular excitations (Ansari et al, 1985). (c) Relaxation pathway proceeds from the active site and runs down the predominant alpha helices.
THE NON-RECURRENT "PERIODIC" TABLE AND THE ELEMENTARY BIFURCATION TREE
Although the discrete quantum aspects of orbital occupancy are periodic, (fig 11 b, c) the properties of successive atoms in the same periods in the table are not exactly, or even approximately, periodic. Successive members of the same group differ significantly in nuclear charge, atomic radius and electron repulsion, resulting in trends which permit interactive bifurcations between their properties. For example the properties of sulphur are significantly different from oxygen, although they are a period apart. The same goes for sodium and potassium through to fluorine and chlorine. When this non-linear non-periodicity complicating the underlying periodicity of the s, p, d and f orbitals is further extended to molecular systems, the parameter space of possible interactions resembles a quantum Mandelbrot set (fig 38) forming an atlas of configurations in which the atomic interactions fig 11(a) and resulting molecular species supporting biogenesis (figs 17, 18) play a pivotal generic role.
Such trends are illustrated in polar and H-bonding properties of hydrides for which H2O is optimal (fig 11(b)), atomic and ionic radii in which the properties of elements like Na and K differ sufficiently to induce distinct H2O bonding structures, and electronegativity, fig 11(c) in which O is even more electronegative than Cl. Such partial, or quasi-periodicity is also illustrated by the intrusion of the transition element d-orbital series between the subsequent s and p series (Moeller et. al.).
 Fig 11: (a) Symmetry-breaking model of selection of bioelements, as an interference interaction between H and CNO, followed by secondary ionic, covalent and catalytic interactions. (b) Boiling points of hydrides illustrate the optimality of H2O as a polar H-bonding medium. (c) Electronegativities illustrate optimality of O and water as a hydride and emphasize the unique role of first row covalent elements C, N, O demonstrated in primitive polymerizations. Atomic and ionic radii also result in a two-way bifurcation of the properties of K, Na, Ca and Mg. Transition elements introduce unique catalytic activities partly through bringing the d-orbital into play.
Fig 11: (a) Symmetry-breaking model of selection of bioelements, as an interference interaction between H and CNO, followed by secondary ionic, covalent and catalytic interactions. (b) Boiling points of hydrides illustrate the optimality of H2O as a polar H-bonding medium. (c) Electronegativities illustrate optimality of O and water as a hydride and emphasize the unique role of first row covalent elements C, N, O demonstrated in primitive polymerizations. Atomic and ionic radii also result in a two-way bifurcation of the properties of K, Na, Ca and Mg. Transition elements introduce unique catalytic activities partly through bringing the d-orbital into play.The stable aspects of quantum orbital interaction in biochemical evolution can be classified into a tree of fundamental bifurcations which distinguish the elements structurally and cause divisions between their properties in interaction. This forms a generative sequence in which the bioelements have key roles (fig 11(a)). Each bifurcation gives rise to a reaction phase with added degrees of freedom and consequently greater interactive complexity. Describing the evolution of interactive chemical quantum structures in terms of funadamental force bifurcations sheds constructive light on the broad categories into which molecular free interaction differentiates and determines both the degrees of freedom and the constraints for development of interactive complexity in bio-molecules. Successive bifurcations are as follows :
1 : Principal Bifurcation : The Covalent Interaction of H with C, N, O.
The central covalent quantum interaction in the table of the elements is between the two-electron 1s orbital and the eight-electron 2sp3 hybrid. This is the fundamental covalent 1-2 shell quantum interaction and the bifurcation through which biocosmology comes into existence. All the members of the CNO group have tetrahedral sp3 bonding geometry and form a graded sequence in electronegativity, from carbon in rough parity with hydrogen to electronegative oxygen, with one and two lone pair orbitals appearing successively in N and O. The resulting 3-D covalent bonds give C, N and O optimal capacity to form complex, diverse polymeric structures. Symmetry is split, because the 1s has only one binding electron state, while the 2sp3 has a series from 4 to 7 with differing energies and varied occupancy, as the nuclear charge increases. The 1s orbital is unique in the generation of the hydrogen bond through the capacity of the bare proton to interact with a lone pair orbital.
Some of the strongest covalent bonds known to chemistry are the multiple-bonds such as -C≡C-, -C≡N, and >C=O. Because of the higher energy of the resulting π-orbitals, these high energy multiple bonds possess a specific type of structural instability, in which one or two π-bonds can open to form lower energy partially σ-bonded heterocyclic and other oligomeric structures. Most of the prebiotic molecular complexity generated by such energy sources can be derived from mutual polymerizations of HC≡CH, HC≡N, and H2C=O, and realated hybrids in association with 'sister' molecules such as urea H2N-CO-NH2. These include purines such as nucleic acid bases adenine and guanine, their pyrimidine complements uracil and cytosine, key sugar types such as glucose and ribose, amino acids, polypeptides, porphyrins etc. They form a core pathway from high energy stability to structurally unstable polymerization, and to complexity, which we will elucidate. The formation of conjugated double and single bonds in these reactions results is delocalized π-orbitals (Pullman and Pullman 1962). Such orbitals in heterocyclic (N-C) rings with conjugated resonance configurations also enable lone pair n → π* and π → π* transitions (Rich and Rajbandry 1976), resulting in photon absorption and electron transfer. These two effects in combination play a key role in many biological processes including photosynthesis, electron transport and bioluminescence.
These molecular interactions, leading to both amino acids and nucleic acid bases such as adenine, can be generated by applying any one of several high-energy sources such as u.v. light, high temperatures (900oC), or spark discharge to mixtures of simple precursors such a HCN, NH3 and H2O (Miller 1953, Miller & Urey 1959, Oro & Kimball 1961, Oro & Kamat 1961, Ponnamperuma, Sagan & Mariner 1963, Lowe, Rees & Markham 1963, Steinman & Cole 1967). Ironically it was discovered in 2021 that the silica glass retorts were also essential reactants in the 1959 experiment! Although the reducing atmosphere of the original Miller-Urey experiment is not now thought to be consistent with the more neutral atmosphere of the early Earth, more recently Johnson et al. (2008) reperformed the analysis of one of the Miller-Urey experiments, showing that a variety of amino acids were formed when steam and reducing gases were present in a more oxidizing context, consistent with volcanism in a more realistic early Earth scenario.
By contrast with hydrocarbons which are able to be fully reduced chains, the silicones such polydimethylsiloxane have an alternating Si-O-Si backbone rendering them unable to enter into the same versatility of structural arrangements.
 Fig 12: Optimality of H2O demonstrated in its varieties of interaction: (a) Dissociation into acidic and basic ions. (b) Hydrogen bonding into the oxygen lone pair. (c) Geometry of the H-bond, (d) Water's high boiling point is a function of H-bonding in which 85% of molecules at room temperature are caught ina bonding lattice. (e) the H-bonded crystal structure of ice. (f) Polar bonding of water molecules with positive and negative ions results in larger hydrated ionic radii, with naked and hydrated ionic radii shown centre.
Fig 12: Optimality of H2O demonstrated in its varieties of interaction: (a) Dissociation into acidic and basic ions. (b) Hydrogen bonding into the oxygen lone pair. (c) Geometry of the H-bond, (d) Water's high boiling point is a function of H-bonding in which 85% of molecules at room temperature are caught ina bonding lattice. (e) the H-bonded crystal structure of ice. (f) Polar bonding of water molecules with positive and negative ions results in larger hydrated ionic radii, with naked and hydrated ionic radii shown centre.
2 : Secondary Splitting between C, N, and O : Electronegativity Bifurcation.
In addition to varying covalent valencies, lone pairs etc., the 8-electron 2sp3 hybrid generates a sequence of elements with increasing electronegativity, fig 11(c), arising from the increasing nuclear charge. This results in a variety of secondary effects in addition to the oxidation parameter, from the polarity bifurcation discussed below, to more subtle effects such as the complementation of -CO2H and -NH2 as generalized organic acidic and basic moieties.
Differential electronegativity results in several coincident bifurcations associated with water structure. A symmetry-breaking occurs between the relatively non-polar C-H bond and the increasingly polar N-H and O-H. This results in phase bifurcation dividing the medium into polar (aqueous) and non-polar phases in association with low-entropy water bonding structures induced around non-polar molecules. This is directly responsible for the development a variety of structures from the membrane in the context of lipid molecules fig 31, to the globular enzyme form and base-stacking of nucleic acids fig 10.
Critical in this process are the optimal properties of water H2O among all molecules, as a polar H-bonding and ionizing medium, making possible in turn polarity interactions, aqueous acid-base bifurcation, ionic solubility and hydrogen bonding. The optimal nature of water as a hydride is illustrated in boiling points Fig 11(b). Water provides several other secondary bifurcations besides polarity. The dissociation 2H2O ↔ H3O+ + OH- lays the foundation for the acid-base bifurcation, while ionic solubility generates anion-cation. Many key properties of proteins and nucleic acids, are derived from water bonding structures in which a counterpoint of H-bonding and phase bifurcation effects occu, determining the form of the alpha helix and nucleotide base pairing. Hydrophilic-non-polar bifurcation is central to the tertiary structures of globular proteins as 'micelles' and hairpins of RNAs, fig 10. The solubility or otherwise of a variety of molecules and ions is derived from the energies and entropies of their induced water-bonding structures. The large diversity of quantum modes in water is demonstrated by its very high specific heat, contrasting with that of proteins (Cochran 1971). Polymerization of nucleotides, amino-acids and sugars all involve dehydration elimination of H2O, giving water a central role in polymer formation. It has also been suggested water is a two phase medium containing quantum-coherent domains, in association with boundaries such as macromoleculaes and membranes (Mae-wan Ho ISIS Report).
 Fig 13: The diversity of snow crystals illustrates the complexity of water bonding structures and their diversity under very slight perturbation of initial conditions (Bentley and Humphries).
Fig 13: The diversity of snow crystals illustrates the complexity of water bonding structures and their diversity under very slight perturbation of initial conditions (Bentley and Humphries).3 : Ionic Bifurcation.
The cations bifurcate in two phases : monovalent-divalent, and series (Na+- K+, Mg2+- Ca2+). Although ions such as K+ and Na+ are succesive alkali metals with similar ionization potentials, their hydrated radii have an inverse relation to their naked radii because the smaller Na+ (r=0.95 A, R=3.6 A) has a higher electric potential than K+ (r=1.33 A, R=3.3 A). Mg2+ (r=0.65 A, R=4.2 A) has a significantly greater hydrated radius and a smaller naked radius than the alkali ions because it carries double the electric charge. Ca2+ (r=1 A, R=4 A) falls closer to Na+. This results in a crossed bifurcation between the two series, in which K+ and Mg2+ become intracellular, with Mg2+ having a pivotal role in polyphosphate activity in nucleic acids , and in RNA transesterifications, while Na+ and Ca2+ tend to be extracellular. Phosphates have been found to have an origin in interstellar dust grains (Turner et al. 2018). Cl- remains a central anion. These bifurcations are the basis of membrane excitability and the maintenance of concentration gradients in the intracellular medium which distinguish the living medium from the environment at large.
 Fig 14: Varieties of pyrophosphate energetics. A series of phosphoric acids, the first three of which are central to biology. Isopentenyl pyrophosphate which is involved in cholesterol synthesis. ATP which is both the cell's universal energetics mediator and one of the nucleotides forming RNA. Glucose-6-phosphate central to glycolysis and the pentose phosphate pathway.
Fig 14: Varieties of pyrophosphate energetics. A series of phosphoric acids, the first three of which are central to biology. Isopentenyl pyrophosphate which is involved in cholesterol synthesis. ATP which is both the cell's universal energetics mediator and one of the nucleotides forming RNA. Glucose-6-phosphate central to glycolysis and the pentose phosphate pathway.
4 : Second and Third Row Lower-energy Covalent Modifiers.
The second-row covalent elements are sub-optimal in their mutual covalent interactions and their interaction with H. Their size is more compatible with interaction with O, forming e.g. SiO32-, PO43- and SO42- ions including crystalline minerals. The pivotal role of oxygen in forming these compound cations and many others such as NO3-, CO32- etc. is a fundamental optimality of the periodic table, since O is the most electronegative dicovalent element (fig 11c).
However in the context of the primary H-CNO interaction, two new generic properties are introduced. PO43- is unique in its capacity to form a series of moderate energy dehydration polymers, both in the form of pyro- and poly-phosphates, and in interaction with other molecules such as sugars. The energy of phosphorylation falls neatly into the weak bond range (30-60 kj/mole) making it suitable for conformational changes. The universality of dehydration as a polymerization mechanism in polynucleotides, polypeptides, polysaccharides and lipids, the involvement of phosphate in adenosine triphosphate (ATP) energetics, ribonucleic acid (RNA) and membrane structure, and the fact that the dehydration mechanism easily recycles, unlike the organic condensing agents, give phosphate optimality as a dehydrating salt. In effect organisms are forms of dehydrated mineral phosphate covalently bonded to and driving a variety of organic CNO-H molecules in both an informational and metabolic process.
This an optimal property of phosphorus as an element, not shared by its lateral or vertical relatives on the periodic table. Silicic acid behaves very differently. Partial dehydration to metasilicic acid typically progresses all the way to silicon dioxide and water. In the solid state silicic acids may condense to form polymeric silicic acids of complex structure. Sulfuric acids are characterized only as far as the highly acidic two-unit pyrosulfuric, as higher members are too unstable to be isolated because of reactions like H2S2O7 → H2SO4 + SO3. Carbonic acid is a weak acid that dissociates HCO3- ↔ CO2 + OH-. Boric acid also dissociates B(OH)3 ↔ O=B-OH + H2O and then forms a tetramer O=B-O-B(OH)-O-B(OH)-O-B=O. Polyborate anions are formed at pH 7-10 if the boron concentration is higher than about 0.025 mol/L so the energetics is significantly different from those of phosphates. Nitric and chloric acids are unstably prone to act as oxidizing agents because of their own electronegativity compounding with that of oxygen.
The function of sulphur and selenium in biosystems highlights a second optimality i relation to oxygen. The lowered energy of oxidation transitions in S particularly S-S ↔ S-H , by comparison with first row elements, gives S a unique role as a mediating mild covalent linkage both in terms of tertiary bonding in proteins theough S-S bridges and low energy respiration and photosynthesis pathways in the evolution of photosynthesis from a one-photon S-based process to the two-photon system required to split H2O. The role of the Fe-S complex in electron transport also plays a pivotal role in primordial electrochemistry. In a final step, selenium mediates certain key transformation states in coded proteins forming the series serine (-OH), cysteine (-SH) and seleno-cysteine (-SeH).
5 : Transition Element Catalysis
Transition elements add key d-orbital effects, forming a catalytic group. Almost all of the transition elements e.g. Mn, Fe, Co, Cu, Zn are essential biological trace elements (Frieden 1972), promote prebiotic syntheses (Kobayashi and Ponnamperuma 1985) and are optimal in their catalytic ligand-forming capacity and valency transitions. Both the Fe2+- Fe3+ transition, and spin-orbit coupling conversion of electrons into the triplet-state in Fe-S complexes occur in electron and oxygen transport (McGlynn et. al. 1964). In oxygen transport as in heamoglobin and myoglobin the central iron aton is Fe2+abut in the cytochromes as an electron carrier it undergoes the Fe2+- Fe3+ transition. Zn2+ by coupling to the PO43- backbone, catalyses RNA polymerization in prebiotic syntheses, occurs in the fingers of regulatory DNA binding proteins which bind to the large nucleic acid groove and remains an essential component of all DNA and RNA polymerases. Other metal atoms such as Mo via Mo(IV)-Mo(VI) oxidative transitions, and Mn which has a spectrum of oxidation states, although biological activity centers on Mn(II) and MN(III), have similar optimal functions.
6: Chirality
A breakthrough in chirality(Ozturk et al. 2023) that is realisable in prebiotic Earthly environments has come with the blindingly obvious discovery of chiral-induced spin selectivity (CISS) – that terrestrial magnetism can affect electrons differentially based on their spin and the effect can result in differential crystallisation of L & R enantiomers of key molecular precursors of nucleic acids, such as RAO (Ozturk, Sasselov & Sutherland 2023). A simulation of a magnetite surface subject to stream leaching magnetised by the Earth's magnetic field where ROA or ribo-aminooxazoline can be separated from the other sugar forms and selectvely concentrated by selective crystallisation on the magnetite surface.

Fig 14b: An evaporative lake with magnetic sediments can accommodate spin-selective processes between an RNA precursor and a magnetized surface. (A) An evaporative lake contains authigenic magnetite sediments magnetized by the Earth's magnetic field. An incoming stream with racemic aminooxazolines scours the muddy material off the surface. As the lake evaporates, D-RAO selectively crystallizes on the magnetic surface. (B) An incoming stream carries the reaction products of racemic glyceraldehyde and 2-aminooxazole: racemic RAO, AAO, XAO, and LAO. Initially, the magnetic sediments are authigenically magnetized and carry a bias along the Earth's field. This natural bias enables the enantioselective crystallization of RAO, which also sterically purifies RAO from the mixture of aminooxazolines. After a small enantiomeric imbalance is induced, sporadic water flow redissolves the RAO crystals, and, in a subsequent dry phase, RAO recrystallizes on the surface with higher enantiomeric excess (ee). As these nearly pure conglomerate crystals accumulate more material, they cover a larger area of the surface and flip the spin of magnetic domains along the chiral molecular axis. This chirality-induced magnetization process increases the magnetization of the surface and allows for a positive feedback loop between the magnetic surface and the chiral crystals. As a result, the surface with higher magnetization induces higher ee for the upcoming crystalliza- tions, and, eventually, enantiopure D-RAO is obtained.
A critical advance has come with the blindingly obvious discovery of chiral-induced spin selectivity (CISS) – that terrestrial magnetism can affect electrons differentially based on their spin and the effect can result in differential crystallisation of L & R enantiomers of key molecular precursors of nucleic acids, such as RAO (Ozturk, Sasselov & Sutherland 2023). This coincides with the occurrence of high-phosphate shallow lakes on the early Earth, exemplified by highly concentrated dissolved phosphate in carbonate-rich "soda" lakes involving phosphorus and nitrogen cycling in Last Chance Lake and Goodenough Lake, Canada (Haas, Sinclair & Catling 2024).
 Fig 14c: Top: Due to the CISS effect, magnetic surfaces with net magnetization can function as chiral agents and be templates for the enantioselective crystallization of RAO from its racemic solution by a spin-controlled process. (b) Selective interaction of chiral molecules with the electron spin can be utilized to magnetize magnetic surfaces. Magnetic surfaces with no net magnetization can be uniformly magnetized by crystallization of enantiopure RAO along the chiral molecular axis. (c) Enantioselective crystallization of chiral molecules on magnetic surfaces can be considered together with the chirality-induced magnetization in a cooperative feedback. With this feedback, a small bias in the natural magnetization of magnetic surfaces due to the geomagnetic field can be amplified, and enantioselective processes controlled by the electron spin can be made highly efficient. Note that racemic RAO is synthesized with three other racemic aminooxazolines (arabinose-AO, xylose-AO, and lyxose-AO), which get excluded in the initial crystallization step due to the relatively low solubility of RAO. Bottom: Propagation of homochirality from a common ribonucleotide precursor, RAO, to the entire prebiotic network is illustrated. D-RAO can be transformed into D-ribonucleotides, forming homochiral RNA. L-peptides can be synthesized through the stereoselective binding of L-amino acids to tRNA analogs composed of Dribonucleotides. This crucial link enables the transfer of homochirality from nucleic acids to peptides. Then, L-peptides form L-enzymes, and by enantioselective catalysis, a homochiral metabolism can be produced.
Fig 14c: Top: Due to the CISS effect, magnetic surfaces with net magnetization can function as chiral agents and be templates for the enantioselective crystallization of RAO from its racemic solution by a spin-controlled process. (b) Selective interaction of chiral molecules with the electron spin can be utilized to magnetize magnetic surfaces. Magnetic surfaces with no net magnetization can be uniformly magnetized by crystallization of enantiopure RAO along the chiral molecular axis. (c) Enantioselective crystallization of chiral molecules on magnetic surfaces can be considered together with the chirality-induced magnetization in a cooperative feedback. With this feedback, a small bias in the natural magnetization of magnetic surfaces due to the geomagnetic field can be amplified, and enantioselective processes controlled by the electron spin can be made highly efficient. Note that racemic RAO is synthesized with three other racemic aminooxazolines (arabinose-AO, xylose-AO, and lyxose-AO), which get excluded in the initial crystallization step due to the relatively low solubility of RAO. Bottom: Propagation of homochirality from a common ribonucleotide precursor, RAO, to the entire prebiotic network is illustrated. D-RAO can be transformed into D-ribonucleotides, forming homochiral RNA. L-peptides can be synthesized through the stereoselective binding of L-amino acids to tRNA analogs composed of Dribonucleotides. This crucial link enables the transfer of homochirality from nucleic acids to peptides. Then, L-peptides form L-enzymes, and by enantioselective catalysis, a homochiral metabolism can be produced.
Since the weak force is also chiral, it has been earkier suggested that weak perturbations to electromagnetic chemical interactions could induce chirality as in fig 15, possibly via spin-polarized electrons (Vester et al. 1959, Ulbricht & Vester 1964). An experiment using L- and R-bromocamphor and right and left-handed longitudinally-polarized electrons from weak force decay resulted differential rates of decay of 3x10-4 suggesting that weak force could have been the basis of chirality (Gibney 2014, Dreiling and Gay 2014). For every type of amino acid found in meteorites there is an excess of the left-handed form over the right-handed of between 2 and 18 per cent - a variety of possible cosmic sources, from supernovae to circularly polarized light have been explored (Chown 2010). One study has suggested that the chirality of the amino acids could be established in the magnetic field of a nascent neutron star from a core-collapse supernova or massive collapsar (leading to a white dwarf). The magnetic field would orient the 14N nuclei, and the alignment of its nuclear spin with respect to those of the electron antineutrinos emitted from the collapsing star would determine the probability of destruction of the 14N nuclei by interactions with the antineutrinos (Famiano et al. 2017). Globius & Blandford (2020) have also proposed that chirality could arise from cosmic rays. Cosmic rays are an abundant form of high-energy radiation that originate from various sources throughout the Universe, including stars and distant galaxies. After hitting the Earth's atmosphere, they eventually degrade into fundamental particles. At ground level, most of the cosmic rays exist only as particles known as muons, unstable particles, existing for 2 millionths of a second, and are magnetically polarized, meaning, on average, muons all share the same magnetic orientation. When muons finally decay, they produce electrons with the same magnetic polarization. This could interact differently with L and R forms of polynucleotides whose sugar backbones spiral with opposing chirality leading to differential mutation rates and viability.
 Fig 15: (a) The perturbing effect of the neutral weak force results in violation of chiral symmetry in electron orbits. Without perturbation (i) the orbits are non-chiral, but the action of Zo results in a perturbing chiral rotation. (b) Autocatalytic symmetry-breaking causes random chiral bifurcation (i).Weak perturbation breaks stability to one chiral form (iii)
Fig 15: (a) The perturbing effect of the neutral weak force results in violation of chiral symmetry in electron orbits. Without perturbation (i) the orbits are non-chiral, but the action of Zo results in a perturbing chiral rotation. (b) Autocatalytic symmetry-breaking causes random chiral bifurcation (i).Weak perturbation breaks stability to one chiral form (iii)Given any symmetry-breaking factor, a chiral system would also be driven by autocatalytic feedbacks involving the competition between incompatible chiral froms. We have seen instances of excesses of L-enantiomers of amino acids on carbonaceous chondrites and noted that aspartic acid will differentially crystallize in enantiomeric forms. The discovery of large excesses of L-aspartic and glutamic acids in the Tagish Lake chondrite confirms astronomical circumstances can lead to the biological forms of chiral biomolecules given a minor symmetry-breaking process such as weak radiation. Proline has also been proposed as a source of chirality. In a sample reaction of autocatalytic chirality, a mixture of compounds containing a small excess of one enantiomer of the amino acid leucine reacted to form pyrimidyl alkanol, also with a small excess of one enantiomer, which then acted as a catalyst in its own formation resulting in a highly enantiomeric product (Soai 1998).
7 : Tertiary Interaction of Mineral Interface.
Both silicates such as kaolinite clays (Strigunkova et. al. 1986) and volcanic magmas (Lavrentiev et. al. 1984) have been the subject of intensive interest as catalytic or information organizing adjuncts to prebiotic evolution. Clays have been proposed as a primitive genetic system and both include adsorbent and catalytic sites (Cairns-Smith 1982, Weiss 1981). Clays also appear to play a key role in stabilizing ribonucleotide polymerization (Ferris 1996). The mineral interface involves crucial processes of selective adsorption, chromatographic migration, and fractional concentration, which may be essential to explain how rich concentrations of nucleotide monomers could have occurred over geologic time scales.
Subsequent interest has focused on borates as stabilizers of the ring structure of sugars including ribose and even more particularly the phase interfaces that result from the weathering of olivine a primordial magnesium iron silicate hybrid (solid solution), which produces redox and alkali-base reaction boundaries capable of supporting primitive metabolisms as far-from-equilibrium systems.
These processes between them constitute the major quantum bifurcations in the free interaction of the elements. They are also the central processes operating in biogenesis. Put together this says the following: The central biogenesis pathways are themselves results of the central interactive quantum bifurcations of symmetry-breaking and its resulting non-linear interactions. While life might conceivably be possible using other combinations of elements possibly at other temperatures and pressures, the clear indications are that cosmic symmetry breaking has induced an optimal interactive process, in which life as we know it has taken the optimal blood-royal route of quantum cosmology.
STRUCTURAL DYNAMICS OF THE CORE POLYMERIZATION PATHWAYS
The initial polymerizations of energetic multiple-bonded monomers in the reaction in figs 16 and 17 form a particularly interesting problem from a quantum-mechanical point of view, because they provide some of the richest examples of growth in quantum-mechanical complexity, in which a relatively small number of simpler entities give rise to increasingly complex structures whose properties cannot be fully predicted from the initial conditions.
H2C=O in aqueous solution gives rise to 4 to 7 carbon sugars, including ribose, as well as branched polysaccharides. HCN gives rise to heterocyclic purine and pyrimidine nucleic acid bases, and in addition several amino acids, polypeptides, porphyrins, and many other types of biomolecule (Lowe et. al. 1963, Calvin 1969, Mizutani et. al. 1975). A similar array of products arises from hybrids such as cyanogen N≡C-C≡N (Schwartz et. al. 1975) and cyanoacetaldehyde N≡C-CH2-H2C=O. Although several of these products, such as the ring polymers adenine (HCN)5 and ribose (H2CO)5 are stable product structures, many of the more complex products, such as particular oligopeptides are metastable or stochastic products of the reaction. These conditions differ markedly from the current biochemical regime in which structurally-stable metabolic pathways are maintained through genetically-coded enzyme catalysis except where recombinational stochasticity is specifically initated as in generation of antibody immuno-diversity.
 Fig 16: Known product structures and pathways in HCN polymerization.
Fig 16: Known product structures and pathways in HCN polymerization.
Since the initial conditions do not contain sufficient information to determine the final products, the system contains many potential outcomes. The lower energy configuration of key products, such as adenine's resonance stabilization, leads to some stable conformations based on free energy. Stochastic indeterminacies in the interaction of simpler molecules lead to multiple branching pathways. Products of increasing complexity such as polypeptides possess increasingly active catalytic potential, which may alter the structural-stability of polymerization to favour certain types of product. The dynamics may trigger a sequence of autocatalytic bifurcations, some of which may result in the formation of attracting molecular products. These reaction pathways are capable of producing a vast variety of complex molecules with generic relationships to key biomolecules, including amino acids, polypeptides, HCN polymers, purines, pyrimidines and porphyrins.
Both HCN and HCHO polymerizations have prominent cyclic products which act as spontaneous end points of polymerization, because cyclization mutually neutralizes reactive moieties. The purines, pyrimidines, ribose and other sugars and porphyrins all display structure consistent with being cyclic terminators. The capacity of polymers for non-periodic primary sequencing gives rise to complex tertiary structures, which are fractal as a result of structure on several overlapping scales from the atom, through local groups, to structures such as a-helices through to global conformation changes. This fractal nature is reflected both in the geometry and the quantum energetics of molecular transformations (Ansari et.al. 1985, Liebovitch & Toth 1991). Substrate form is dependent firstly on local active sites, and in turn on the global tertiary structure of catalytic molecules.
 Fig 17: (a) One of several synthesis pathways for pyrimidines. (b) Sample HCHO polymerization routes. Phosphorylation of the oligo-aldehydes causes the reaction to favour ribose. (Eschenmoser 1992).
Fig 17: (a) One of several synthesis pathways for pyrimidines. (b) Sample HCHO polymerization routes. Phosphorylation of the oligo-aldehydes causes the reaction to favour ribose. (Eschenmoser 1992).
The central role of HCHO as a polymerising source of carbohydrates leading to both ribose, the backbone of nucleic acids and glucose, key in energetic metabolism, and its cosmological evidence in star-forming nebulae such as Orion is complemented by a similar key role in all living systems (Burgos-Barragan et al. 2017, Pietzke, Meiser & Vazquez 2019). Formaldehyde is commonly regarded as a toxic contaminant that can cause cancer or kidney failure and blindness in acute methanol poisoning when methanol is converted to formaldehyde. rather than the acetaldehyde of ethyl alcohol. However, formaldehyde, generated from the folic acid backbone and then metabolised to formate, is the central substrate in the 1-carbon cycle, key for the formation of both amino acids and nucleic acids through 1-C transfers. HCHO thus remains in all of us an essential player in the generation of complex molecules and the ability of genetic systems to replicate and survive. Because of its multivalent fecundity, it is also highly toxic, gluing up the molecular and genetic machinery with additional carbohydrate bonds, so living systems also invoke both detoxification enzymes to mop it up and genetic repair enzymes that can correct formaldehyde-induced damage to DNA to keep the entire balance sheet sustainable.
Although the first syntheses produced the purines adenine and guanine readily, cytosine and uracil, the complementary pyrimidine bases making up the other half of the pair A-U and G-C, however Stanley Miller, forty three years after his original pioneering experiment in spark synthesis, with Michael Robertson, discovered a way for the primordial pond to make them in high yield. Although urea is produced in Miller's original experimental setup, it never reaches a high enough concentration. When he added more urea, it reacted with cyanoacetaldehyde, another by-product of the spark synthesis, churning out vast amounts of the two bases. Urea would have been able to reach high enough concentrations as shallow pools of water on the Earth's surface evaporated. (Cohen 1996, Horgan 1996).
 Fig 18: The stabilization of ribose and other 5-carbon sugars by borates protects them against reactive degradation into brown random polymers.
Fig 18: The stabilization of ribose and other 5-carbon sugars by borates protects them against reactive degradation into brown random polymers.
Eschenmoser (1992) has found that glyceraldehyde phosphate in the presence of HCHO will produce 5-carbon sugars with up to 33% ribose. In the absence of HCHO the reaction tends to produce 6-carbon sugars. The phosphate-induced reaction is key here because RNA, ATP and glycolysis all involve phosphate dehydration energy. This indicates a specific link to phosphate energy primordial to the formation of oligonucleotides and even ribose.
Building ATP's complex chemical structure from scratch is energy intensive and requires six separate ATP-driven steps. ATP was likely quite scarce. This means that some other compound may have played a central role in the conversion of ADP to ATP at this stage of evolution. The most likely candidate, Lane and colleagues (Pinna et al. 2022) believed, was the two-carbon compound acetyl phosphate (AcP), which functions today in both bacteria and archaea as a metabolic intermediate. AcP has been shown to phosphorylate ADP to ATP in water in the presence of iron ions, but a host of questions remained after that demonstration, including whether other small molecules might work as well, whether AcP is specific for ADP or instead could function just as well with diphosphates of other nucleosides (such as guanosine or cytosine), and whether iron is unique in its ability to catalyze ADP phosphorylation in water. They tested the ability of other ions and minerals to catalyze ATP formation in water; none were nearly as effective as iron. Next, they tested a panel of other small organic molecules for their ability to phosphorylate ADP; none were as effective as AcP, and only one other (carbamoyl phosphate) had any significant activity at all. Finally, they showed that none of the other nucleoside diphosphates accepted a phosphate from AcP.
 Fig 18b: Potential mechanism of ADP phosphorylation in water.
Fig 18b: Potential mechanism of ADP phosphorylation in water.
Benner and coworkers have discovered that the presence of borate stabilizes 5-carbon sugars in their ring form sequestering their active preventing the active C=O in the open ended-forms undergoing random polymerization into the brown tars that would prevent a nucleotide metabolism form forming (Ricardo et al. 2004). The involvement of borates has a wider cosmological basis with highly boron-enriched clays having been detected on the Martian surface (Stephenson J., Hallis L.,  Nagashima K., Freeland S. (2013) Boron Enrichment in Martian Clay Plos One 8/6 e64624). Powner and Sutherland (2010) have also investigated interconversion of sugar bases between ribo and arabino nucleotide intermediates.
Nagashima K., Freeland S. (2013) Boron Enrichment in Martian Clay Plos One 8/6 e64624). Powner and Sutherland (2010) have also investigated interconversion of sugar bases between ribo and arabino nucleotide intermediates.
Fig 19: Sugars produced in an experimental simulation of irradiated pre-cometary ice.
In a more recent experiment recreating conditions substantial quantities of ribose and a diversity of structurally related sugar molecules such as arabinose, xylose, and lyxose in the room-temperature organic residues of photo-processed interstellar ice analogs initially composed of H2O, CH3OH, and NH3, suggesting that the generation of numerous sugar molecules, including ribose, may arise from photochemical and thermal treatment of cosmic ices in the late stages of the solar nebula (Meinert et al. 2016). A more recent study has also found deoxyribose and analogs (Nuevo et al. 2018).
 Fig 20: (a) MgATP-complex illustrates linkage between primal stability structures. Cyclic pentamers of HCN (adenine) and HCHO (ribose) are linked by phosphate dehydration, stabilized by cation and water structures. (b) Heterocyclic form of heme. Porphyrins have also been detected in primal syntheses. (c) Nucleophilic attack of adenine N9 on ribose.
Fig 20: (a) MgATP-complex illustrates linkage between primal stability structures. Cyclic pentamers of HCN (adenine) and HCHO (ribose) are linked by phosphate dehydration, stabilized by cation and water structures. (b) Heterocyclic form of heme. Porphyrins have also been detected in primal syntheses. (c) Nucleophilic attack of adenine N9 on ribose.
COSMOLOGICAL PATHWAYS TO NUCLEIC ACIDS
In 1981 Francis Crick commented that 'the origin of life appears to be almost a miracle, so many are the conditions which would have to be satisfied to get it going." (Horgan 1996) Now, several findings bolster the dominant theory of genesis - that life began in an era in which RNA was both the genetic and catalytic basis - the RNA era (Gilbert 1986, Benner et.al., Joyce and Szostak 2018) in which simple replication and 'enzymatic' process based purely on RNA catalysis established evolutionary biochemistry.
The general outlines are clear. Ribose, unlike the deoxyribose in DNA, has plausible prebiotic syntheses. RNA's capacity to both form double-helices, like DNA and to also three-dimensional tertiary structures similar to proteins through base-backbone bonding to ribose fig 10(a), causes RNA to have both genetic and catalytic capacity. Simple biological RNAs have been demonstrated to have autocatalytic self-assembling capacity. The catalytic activity of polynucleotides, hinges on various forms of proton transfer 25(a,b,c) (Pace and Marsh 1985), in particular transesterification.
The essential core of the protein-assembling ribosome remains RNA as does the signal recognition particle which shepherds nascent proteins through the membrane. The ancient fossil nucleotide coenzymes including ATP, NAD, coenzyme-A and Vitamin B12 are all ribonucleotides. Eucaryote organisms continue to have a massive commitment to RNA processing within the nucleus, including the use of many small small nuclear ribonucleotides or snurps involved in RNA splicing. This suggests eucaryotes have never fully transferred from an RNA-based metabolism. Reverse transcriptases also remain ubiquitous and essential for such basic functions as telomere extension, and have a common evolutionary tree, giving retrotransposons and retroviruses a potentially ancient origin in the commonality of the RNA era.
There is still debate about whether RNA was actually the primordial genetic molecule and other hybrid molecules such as peptide-nucleic acids which use peptide rather than sugar linkages also have genetic potential and plausible prebiotic status (Nelson et. al.), however it is clear RNA itself has generic status as a cosmological molecular structure on several grounds. Adenine is a principal thermodynamic product of HCN polymerization in industrial yields. All of A, G, U and C now have prebiotice status as favoured products of such reactions. Ribose is an optimal sugar conformationally in terms of permitting complementary double helix formation, and has a synthesis route from glyceraldehyde phosphate. The complementations of A-U and G-C posses a type of structural optimality among the bases. The heterocyclic polymers are restricted in their variety by the positions of N atoms required by the polymerization process. The tautomeric states of A, U, G and C indicate AU and GC may be optimal for base-pairing among close prebiotic variants.
 Fig 20b: Synthesis of purine bases from u.v. irradiated formamide (Barks et al. 2010).
Fig 20b: Synthesis of purine bases from u.v. irradiated formamide (Barks et al. 2010).
The nucleotide unit, as exemplified in ATP consists of a direct concatenation of key products of HCN and HCHO polymerizations. Adenine and ribose are the cyclic pentamers of HCN and HCHO linked via dehydration to a dehydrating oligo-phosphate giving it the status of a generic structure, fig 20(a) stabilized by water and Mg2+, Positive ions also play an important role in stabilizing mono- and oligo-nucleotides. It was originaly synthesized under primitive conditions by Ponnamperuma et. al. (1963). Mg2+ ions are also bound to transfer RNA and play a critical role in transesterification, balancing the negative phosphates. The fact that the polymerizing phosphodiester bond results from the removal of H2O from phosphate suggests that phosphate was the active moiety linking of the base-sugar-phosphate complex, fig 20(c) and thus drove the entire formation of nucliec acids.
RNA proved difficult for a time to induce into complementary replication in enzyme-free systems, but its relative difficulty of synthesis may be essential to its function. It is necessary that RNA be thermodynamically unstable, or life could not exist dynamically but would 'crystalize' all the way to non-genetic polymers. A variety of partial model systems of complementary replication have been realized by Orgel and his coworkers, however instabilities in polymerization have hindered experimental enzyme-free complementary polymerization of RNAs (Orgel 1992). It is clear that a regime of polynucleotide chemistry would have to have occurred stably over evolutionary time scales for an RNA-based form of life to evolve to the point where it had established translation and captured metabolic synthetic pathways.


Fig 21: Left: Pyrimidine ribonucleotide synthesis routes (Powner et. al 2007). Right: 2020 one pot process leading to replicating RNA-DNA UCdAdI alphabet (Xu et al. 2020)
In a fundamental breathrough, Powner, Gerland & Sutherland (2009) discovered that pyrimidine nucleotides can be readily synthesized from simple prebiotic molecules bypassing the more difficult routes depending on synthesizing ribose, bases independently and trying to then attach them to phosphate. In fact phosphate was pivotal in producing new intermediates which would then in good yield polymerize to nucleotides. In 2010 his colleagues have proposed one-pot pathways to both purine and pyrimidine nucleotides (Powner, Sutherland and Szostack 2010).
In 2020 Sutherland's team expanded this to an ingenious single-phase process capable of generating both U and C and deoxy purines dA and dI (inosine) which form two pairs of complementary binding purines and pyrimidines thus forming in one step a four member alphabet for complementary replication(Xu et al. 2020, Le Vay & Mutschler 2020). This is discussed in greater detail below.
Ferris reported (1996) that he had found a means by which the first large chains could have been forged. When his team added montmorillonite, a positively charged clay believed to be plentiful on the young Earth, to a solution of negatively charged adenine nucleotides, it spawned RNA 10-15 nucleotides long. If these chains, which cling to the surface of the clay, were then repeatedly 'fed" more nucleotides by washing them with the solution, they grew up to 55 nucleotides long. Ferris notes the clay gets RNA off the hook of having to take on the tasks of information storage and catalysis in one fell swoop. It would catalyse RNA synthesis, stocking pools with a large range of RNA strands that, as Szostak and others have shown, would evolve a catalytic capacity of their own. (Horgan 1996). Thus complementary replication can come into existence after a phase of single-stranded polymerization has given rise to a fractal RNA environment with a diverse array of oligomeric and polymeric structures, which in turn feedback autocatalytically on replication and monomer synthesis.
Barks et al. (2010) have also shown that guanine, adenine, and hypoxanthine can be produced from formamide in a single model prebiotic reaction, if formamide is subjected to UV irradiation during heating. Sutherland and co-workers have also explored plausible pathways for formation of ribose. Ritson and Sutherland (2012) show that the sugar building blocks for RNA synthesis - glycolaldehyde and glyceraldehyde can be formed from hydrogen cyanide by ultraviolet irradiation in the presence of cyanometallates.
 Fig 21b: The FaPy pathway to purine nucleotides gives much higher selectivity and better yields than attempting to combine purines and sugars.
Fig 21b: The FaPy pathway to purine nucleotides gives much higher selectivity and better yields than attempting to combine purines and sugars.
Thomas Carell and his team (Becker et al. 2016) discovered an alternative route to purine nucleotides, with much better yields than the Orgel route via fully formed purines and sugars, instead reacting formamidopyrimidines with sugars providing the natural N-9 nucleosides with high selectivity and in good yields (60%). The formamidopyrimidines are in turn available from formic acid and aminopyrimidines, which are in turn available from prebiotic molecules that were also detected during the Rosetta comet mission. This nucleoside formation pathway can be fused to sugar-forming reactions to produce pentosides, providing a plausible scenario of how purine nucleosides may have formed under prebiotic conditions.
Carrel has reported in Oct 2018 that he and his colleagues have discovered a simple set of reactions that could have given rise to all four RNA bases. To make the pyrimidines, Carell started with cyanoacetylene and hydroxylamine, which react to form amino-isoxazoles. These, in turn, react with urea, to form compounds that then react with ribose to make s set of intermediate compounds. In the presence of sulfur-containing thiols and trace amounts of iron or nickel salts, these intermediates transform into the pyrimidines cytosine and uracil. As a bonus, this last reaction is triggered when the metals in the salts harbor extra positive charges, which is precisely what occurs in the final step in a similar molecular cascade that produces the purines, adenine and guanine. Pivotally, the process that leads to all four nucleotides works in one pot.
Others have concentrated on the problems of achieving standard 3'-5' linkages in oligoribonucleotides. Engelhart et al. (2013) show that the 2'-5' 3'-5' backbone heterogeneity in prebiotic syntheses of RNA is compatible with RNA folding into defined three-dimensional structures that retain molecular recognition and catalytic properties. Another group of Sutherland and coworkers (Bowler et al. 2013) has discovered that the 2'-hydroxyl group of oligoribonucleotide-3'-phosphates can be chemoselectively acetylated in water under prebiotically credible conditions, which allows rapid and efficient template-directed ligation. This suggests a prebiotic route from ribonucleoside-2',3'- cyclic phosphates to predominantly 3',5'-linked RNA via partially 2'-O-acetylated RNA. Adamala and Szostak (2013) have also shown that citrate stabilizes fatty acid vesicles in the presence of Mg++ ions while allowing RNA copying to proceed solving how RNA replication might be able to take place in protocells, which would otherwise be destabilized by the Mg++ concentrations required for RNA ligation.
 Fig 22: Hypothetical pathways to both purine and pyrimidine nucleotides from the above paper
Fig 22: Hypothetical pathways to both purine and pyrimidine nucleotides from the above paper
A central scenario out of many, including volcanic hot pools, and hydrothermal vents, is the three-phase boundary of a phosphate-rich, clay shore line under tidal or weather-related variations in a pool in which the margin is reversibly dehydrated e.g. by sun-drying. Both clays and volcanic basalts have been cited as possible mineral interfaces. Precipitated phosphate at 37o, leads to pyrophosphate formation and hence phosphate bond energy (Hermes-Lima 1990). Since the energy for nucleotide polymerization is driven by H2O removal, reversible dehydration of a medium containing phosphate, bases and sugars provides one of the most direct and simple routes to polynucleotide formation. Toner and Catling (2019) propose an environment similar to carbonate-rich lakes found today in places like Lake Magadi in Kenya as a source of high-soluble phosphate.
In a 2012 paper, Benner at al have set out a two-phase process involving dehydrating phase possibly involving non-polar solvents such as formamide which could have been common on the primitie earth with aqueous phases, leading to the formation of oligomeric RNAs (Benner et al. 2012). The dehydrating phase in a less polar solvent would both serve to promote polyphosphate energetics and avoid the continuing hydrolysis that results from the reactivty of H2O for molecules critical to the formation an d maintenance of RNA.
d maintenance of RNA.
Fig 23: Benner et al.'s scheme for alternating dehydrating and aqueous phase oligomerization of nucleotides.
Sutherland and co-workers (Patel et al 2015) have shown that precursors of ribonucleotides, amino acids and lipids can all be derived by the reductive homologation of hydrogen cyanide and some of its derivatives, and thus that all the cellular subsystems could have arisen simultaneously through common chemistry. The key reaction steps are driven by ultraviolet light, use hydrogen sulfide as the reductant and can be accelerated by Cu(I)-Cu(II) photoredox cycling with experimental support for good yields of molecular types key to biogenesis without the plethora of low yield products found in previous sytheses, such as the Miller-Urey spark discharge, the formose reaction and Oro's synthesis of purine nuceobases from NH4CN.
previous sytheses, such as the Miller-Urey spark discharge, the formose reaction and Oro's synthesis of purine nuceobases from NH4CN.
Fig 23b: Yields for parts of the reaction network shown in fig 23c.
Rather than a single reaction phase, this is envisaged geochemically as an overall reaction network developing over time in separate streams and pools, according to a dynamic flow chemistry scheme. The various products would be synthesized by subtle variations in the flow-chemistry history of the streams and the order in which they merged or ran into pools, to bring together all the essential molecules for biogenesis, through fluid mixing due to preciptation.
In 2017, Powner Szostak and coworkers discovered complementary synthesis pathwyas to the 8-oxo variants of the purines adenine and guanine leading from the same precursor glycoaldehyde interacting with either cyanamide to form primidines or thiocyaic acid to generate oxo-purines. Powner is now working on how the oxo variants could be converted to the more reduced natural H-containing forms. This represents a major breakthrough in understanding the central synthesis pathway of biogenesis.


Fig 23c: Left: Proposed synthesis network leading to biogenesis chemistry from Patel et al (2015).
Right: Complementary actual syntheses to both pyrimidines and 8-oxo purines Stairs et al. (2017)
Hud and co-workers (Chen et al. 2014) report a one-pot model prebiotic reaction between a pyrimidine nucleobase (2,4,6-triaminopyrimidine, or TAP) and ribose, which produces TAP- ribose conjugates in high yield (60−90%) see fig 23d. When cyanuric acid (CA), a plausible ancestral nucleobase, is mixed with a crude TAP+ribose reaction mixture, micrometer-length supramolecular, noncovalent assemblies are formed. The robustness of this reaction is unique, going all the way to high yield polymers. That said, the methuselah molecule needs to be thermodynamically critical or unstable to ensure the reaction doesn't irreversiibly go to dynamic self-extinction. The reaction, while significantly different from RNA has been proposed to form a potential precursor to RNA.

Fig 23c2: Synthesis of both ribo-pyrimidines U, C and complementary deoxyribo-purines dA, dI.
In 2020, John Sutherland's team (Xu et al. 2020) has come up with what could be the most significant break-through so far. They have discovered that the pyrimidine sythesis described in fig 21 can produce alternative pathways which lead to deoxy-adenosine and deoxy-inosine.
They re-examined compounds produced as intermediates in the previously established reaction network that synthesizes U and C. and identified a pathway in which a key intermediate of pyrimidine-nucleoside synthesis, ribo-aminooxazoline (RAO), can also be converted into two purine DNA nucleosides, deoxyadenosine (dA) and deoxyinosine (dI). Crucially, these DNA nucleosides can form base pairs with U and C. The four nucleosides — U, C, dA and dI — therefore constitute a complete 'alphabet' that could have encoded genetic information in nucleic acids in a prebiotic RNA–DNA world. Importantly, the synthesis of dA and dI can occur in parallel with that of U and C, producing mixtures of the four products in yields and ratios suitable for the construction of a genetic system. Such chimeric nucleotide molecules have previously been proposed (Gavette et al. 2016).
Under certain reaction conditions, U and C can survive only in the presence of the thioanhydropurine compounds that act as direct precursors of dA and dI. The purine synthesis is highly selective for the chiral enantiomers and other isomers of nucleosides observed in modern biology. The enantioselectivity derives from RAO, which can crystallise as a single enantiomer from reactions in which the starting materials consist of an almost equal mixture of enantiomers. This provides a solution to the problem of chirality (Hein, Tse & Blackmond 2011) which remains in the two syntheses discussed below.
Nucleoside synthesis can also lead to products in which the nucleoside's base is attached to the sugar in the wrong orientation. In the described synthetic pathway, a UV-induced chemical reduction occurs that leads to the strikingly selective destruction of these unwanted by-products, ultimately producing only the biologically relevant isomers of the purines. Given that early Earth was highly irradiated by UV, the remarkable selectivity of this reaction suggests a possible mechanism by which the total pool of potential nucleic-acid isomers was reduced to the subset of isomers observed today in nature.
Further discussion of underlying research related to this area can be found in Sutherland (2017) in which the underlying milecular mechanisms are explored. Sutherland notes: "If the starting materials were irrefutably primordial and the end result happened to bear an uncanny resemblance to extant biology — for what turned out to be purely chemical reasons, albeit elegantly subtle ones — then it could be a recapitulation of the way that natural life originated. We are not yet close to achieving this end, but recent results suggest that we may have nearly finished the first phase: the beginning".


Fig 23c2 Left: Synthesis using pure ribose to form both pyrimidines and purines (Becker et al. 2019). Right: Deoxynucleotide synthesis in which ribose is produced last (Teichert, Kruse & Trapp 2019).
The Sutherland team's pathway can be compared with other recent ones in which purines and pyrimidines are formed using pure ribose (Becker et al. 2019) and that of Teichert, Kruse & Trapp (2019) in which DNA nucleotides are formed first, with ribose being formed at the end of the pathway. Although these give rise to pure RNA and DNA nucleotides respectively, again consistent with the diea that DNA could have been a nucleotide polymer at the outset, and the reactions may have the potential to form A, U, G and C monomes, the chriality problem of the sugars remains in both.
For a review of aboitic nucleotide synthesis see Yadav, Kumar & Krishnamurthy (2020). In related papers Ruiz-Mirazo, Briones & de la Escosura (2014) present a very comprehensive review of prebiotic systems chemistry, Pascal, Pross & Sutherland (2013), explore the kinetic and thermodynamic basis of these steps, and Laneuville, Kameya & Cleaves (2018), explore the rich properties of the primordial nitrogen cycle.


Fig 23d: Top Left: The Hud polymerization discussed above Lower. Model systems for polypeptide autocatalysis and colloidal clusters discussed below.
Right: Assembly theory provides a partial explanation for certain aspects of molecular oligomerisation (Sharma et al.(2023)
.
Matthew Powner's group has more reecntly discovered a set of sulphur-based molecules that likely would have been present on early Earth and shown how they readily link individual amino acids to amino acid precursors, called aminonitriles, forming dipeptides (Canavelli, Islam & Powner 2019). Because these reactions take place in water and work with all the amino acids found in living organisms, they offer a plausible route to how the first proteins may have formed. Donna Blackmond, and her colleagues tested two of Powner's sulphur compounds to see whether the catalysts were sensitive to chirality as they formed dipeptides. They tested two of Powner's sulphur compounds to see whether they were sensitive to chirality. The catalysts created about four times as many "heterochiral" dipeptides — pairing a left-handed amino acid (L) with a right-handed (D) one—as fully chiral products. In a series of experiments, they started with skewed proportions of L and D amino acids—for example, 60% Ls and 40% Ds. The L,D and D,L heterochiral dipeptides formed most quickly, and as they did they pulled equal numbers of L and D amino acids out of the mix. Because of the baseline bias, eventually a predominance of Ls remained in the pool of unreacted amino acids, raising the likelihood of forming fully lefthanded dipeptides. The team found that heterochiral dipeptides precipitate out of a solution more quickly than homochiral ones, speeding the way to a relative abundance of either homochiral L,L or D,D pairs, depending the starting mix (Deng, Yu & Blackmond 2024).
Fig 23d2: Left: Synthesis cycle from Powner's group. Reaction profiles in Blackmond's group under different L and R reactant proportions.
MOLECULAR SYMBIOSIS, PROTOCELLS AND THE MEMBRANE
Interest in protocells as a basis for coordinated selection of early replicating nucleotides, or abiotic molecular stability structures, forms a key research avenue throughout prebiotic research (Przybylski & Fox 1986, Deamer & Pashley 1989, Dworkin et al. 2001, Adamala and Szostak 2013, Joyce and Szostak 2018).
When I first became involved in exploring the origins of life, I set up a very simple experiment following some of the primal synthesis. I generated HCN by adding acid topotassium cyanide, a risky operation in my customized mathematics office room under a fume chamber fitted into the window and applied it to various mixtures involving NH3 and sometimes HCHO. While I didn't have the analytical apparatus to investigate the products, which I would have liked to have done as a biochemical reactor using chromatography and mass spectrometry, some of the reactions came up with fascinating membranous micorcellular structures, as shown in fig 23e. As I was also a free-lance myclologist, I compared thes with the spores of a psilocybe species I was studying and found they were on the same scale of size as small eucaryote spore cells. This indicates that prebiotic pathways can not only lead to molecules associated with life, but also to membranous structures that involve moleules including oligopeptides and oligonucleotides in association with membranous structures formed by lipid molecules such as fatty acids.

Fig 23e: Left and centre: Microcellular formations generated by the author from HCN and NH3 over H2O sometimes with HCHO.
Right: Spores of a psilocybe species at the same magnification for size comparison (King).
In 2019, investigations were reported (Cornell et al. 2019) that both go toward explaining how amino acids could have stabilized membranes in unfavorable environments and demonstrate how the individual building blocks of cellular structures -- membranes, proteins and RNA -- could have co-localized within watery environments on the ancient Earth. Roy Black who wanted to see why cells, which are made up of very different types of structures with totally different types of building blocks, for which it has never been clear why they would come together in a functional way, teamed up with Sarah Keller, an expert on membranes. The team previously showed that the building blocks of RNA preferentially attach to fatty acid membranes and, surprisingly, also stabilize the fragile membranes against detrimental effects of salt, a common compound on Earth past and present. The illustrations in fig 23f show how amino acids can stabilize membranous structures created by molecules such as decanoic acid, resulting in multi-layer vessicles.

Fig 23f: (Left) Amino acids showing binding affinity with decanoic acid in aqueous solution. (Centre) Micelles stabilized by serine and glycine
under varying
NaCl and Mg2+ concentrations. (Right) Multi-membran micelles produced.
THE LOST CITY CHEMICAL GARDEN OF EDEN: A key potential context for the formation of biogenic molecular reaction systems has been the discovery of 'lost-city' sea-floor vents. Unlike the volcanically induced high-temperature black smokers, these formations are based on the chemical weathering interaction of olivine with sea water. Olivine is a cosmologically abundant primordial geochemical with the formula (Mg2+, Fe2+)2SiO4 forming a solid solution of the two component salts forming a spectrum from Mg dominant forsterite to iron dominant fayalite. It is cosmologicaly primordial and abundant. As well as being a component of primal magmas in the Earth's crust, Mg-rich olivine has been discovered in meteorites, the Moon, Mars, in comets, on asteroid 25143 Itokawa, and falling into an infant star in the Orion nebula. Meteorite occurrences include chondrites, collections of debris from the early solar system and pallasites, mixes of iron-nickel and olivine. Carbonaceous chondrites such as the Murchison meteorite contain a rich variety of prebiotic molecules.

Fig 24: (a) Lost City vents. (b) Lost city vent pore simulations under thermal gradients concentrating nucleotides 1200-fold (Baaske et al.) (c) Olivine grains in Papakolea Beach sand Hawaii (d) Star in the belt of Orion nebula detected by the Spitzer telescope to have infalling olivine rain. (e) The spectrum of forsterite at the magnesium end of the olivine spectrum. (f) The asteroid Itokawa found to contain olivine.
In "lost-city" vents, olivine has been found to form carbonate columns with pores which have been demonstrated to be able to concentrate organic and in particular nucleotide molecules by a factor of over 1000, bringing them up to molar concentrations where a reactive metabolism and informational replication could be sustained (Baaske et al. 2007).
Serpentinization occurs when rocks derived from the upper mantle (rich in olivine), especially at places where the lithosphere is spreading such as mid-ocean ridges, are exposed to ocean water, which percolates down fractures several km to react with rocks beneath the sea floor. This exothermic reaction, combined with geothermal heat, warms the circulating fluid to ~150oC, generating a buoyant alkaline (pH 9-11, note magnesium hydroxide in the above equation) mineral-laden hydrothermal fluid, originally sourced from the ocean, that rises up to the sea floor and exhales at 70-90oC. In the Fe-rich acidic ocean of the early Earth with a higher CO2 concentration, the alkaline weathering "serpentization" of olivine 3Mg3Fe(SiO4 )2 + 7H2O → 3Mg3Si2O5 + Fe3O4 + H2 forms a primal phase interface leading to the idea that it could support varied forms of metabolism without the need for cell walls to maintain concentration gradients. Abiogenic C1 - C4 hydrocarbons can still be detected emerging from "lost city" vents (Proskurowski et al. 2008) consistent with a Fischer-Tropsch synthesis (2n+1)H2 + nCO → CnH2n+2 + nH2O by such approximate reactions as:
(Mg,Fe)2SiO4 + H2O + C → Mg3SiO5(OH)4 + Mg(OH)2 + Fe3O4 + H2 + CH4 + C2-C5
The alkaline vent interface provides a solution to the paradox besetting the origin of life - which came first replication or metabolism? As the alkaline vents effectively provide a membrane-like interface between phases, they promote stable far-from-equilibrium conditions able to sustain dissipative metabolic pathways at the same time as providing a means to concentrate and energize oligomerization of nucleotides. The redox and H+ ion energy interface of Lost City vents has been proposed to be pivotal to the origin of metabolism and coincides with the metabolism of LUCA our last universal common ancestor.
A study has been undertaken to develop a prebiotic chemical reactor based on the chemical dynamics of lost city vents (Hershey et al. 2014). A second study has confirmed using transient compartmentalization of populations of RNAs in a 'fizzing' bubble filled medium, that small RNA ribzymes replicating under rattack from smaller parasitic RNAs can maintain their replication because the partitioning provdes a level of selection to eliminate bubbles which hae been compromized by the parasites. Transient compartmentalization in natural, abiological compartments could thus have allowed life to take hold (Matsumura et sl. 2016).
SELF-REPLICATING SYSTEMS AND THERMODYNAMICS
The planetary interface forms a far-from-equilibrium environment in which life is an open system where there is an incoming source of (radiant solar) energy, which is ultimately being dissipated into the environment in the land, atmosphere and oceans as heat. Although entropy must increase over time in an isolated "closed" system, an "open" system can keep its entropy low — that is, divide energy unevenly among its atoms — by greatly increasing the entropy of its surroundings. In the 1960s, the Belgian physicist Ilya Prigogine made progress on predicting the behavior of open systems weakly driven by external energy sources (for which he won the 1977 Nobel Prize in chemistry). But the behavior of systems that are far from equilibrium, which are connected to the outside environment and strongly driven by external sources of energy, could not be predicted. In the 1990s, Jarzynski and Crooks showed that the entropy produced by a thermodynamic process, corresponds to the probability that the atoms will undergo that process divided by their probability of undergoing the reverse process. As entropy production increases, so does this ratio: A system's behavior becomes more and more "irreversible." The formula can in principle be applied to any thermodynamic process, no matter how far from equilibrium.
Using their formulation, Jeremy England (2013) derived a generalization of the second law of thermodynamics that holds for systems of particles strongly driven by an external energy source such as an electromagnetic wave, that can dump heat into a surrounding bath. This class of systems includes all living things. He then determined how such systems tend to evolve over time as they increase their irreversibility – the more likely evolutionary outcomes are the ones that absorb and dissipate more energy from the environment's external drives on the way to getting there, giving a general thermodynamic rule for the evolution of such dissipative systems that they should tend over time to arrange themselves to resonate better and better with the sources of mechanical, electromagnetic or chemical work in their environments, leading to the notion that the self-organizing photosynthetic biosphere is a statistical consequence of thermodynamics. Replication of a non-equilibrium system is a principal way of incresing this energy transfer. In his 2013 paper he shows for example that RNA replication fits within the limits of the theory. The same principle has been demonstrated both in vortices in turbulent fluids spontaneously replicating themselves by drawing energy from shear in the surrounding fluid (Marcus et al. 2013) and in theoretical models of self-replicating colloidal clusters (Zeravic and Brenner 2014).
Beyond the thermodynamic model, other work has also demonstrated theoretical autocatalytic systems of polypeptides identified only by their hydrophilic or hydrophobic moeties, which are pivotal in determining the three dimensional structure of biological protein folding. In the model system, both self-catalytic and mutually autocatalytic pairs of polymers can be generated, demonstrating that proteins could also have the capacity for forms of self-organizing replication (Guseva et al. 2017).
DIVERSE HORIZONS OF THE RNA EPOCH
The 2020 research from Sutherland's team shows us that what was previously considered to be the RNA era may have actually been an era of interclaced ribo-U and ribo-C nucleotides coupling to deoxyribo-A and deoxyribo-I nucleotides formining hybrid RNA-DNApolymers with properties somewhere between those of RNA and DNA still with a capacity to form catalytic structures but with greater dounble helical stability than RNA. These discoveries will require further work to see if pre-biotic polymerizations of such bases are possible and if so, what their properties actually are.
A field of RNA-era research has developed from the discovery of spontaneous splicing of RNAs in living systems by Tom Cech and the demonstrated capacity of such RNAs to function as catalysts in transesterifications and the work of Jack Szostack's teams in selective RNA catalysis (Cech 1986a). This immediately made the idea of the RNA world before proteins a natural hypothesis. This work has grown with artificial selective evolutionary studies, culminating with the development of a ribozyme which is capable of high fidelity complementary replication of short RNA oligomers of arbitrary sequence (Johnston et.al. 2001). This has become a turning point in the credibility and maturity of the RNA world as a precursor to DNA-based life which can develop as an autonomous molecular system.
The model has been extended to others for RNA-based error-correction, synthetases and the ribosome (Bass and Cech 1984, Cech 1986b, Zany and Cech 1986, Garriga et. al. 1986, Weiner and Maizels 1987). Modified ribozymes are capable of acting as polymerases which can replicate complements to subsections of themselves (Green et. al. 1990, Doudna et. al. 1991). The active cleavage site of the Tetrahymena group 1 intron is catalysed by metal ions, including Mg++ and Mn++, confirming that, derived from their acidic phsophate backbone, ribozymes function essentially as metallo-enzymes (Shan 1999). They are thus adapted to a mineral-rich environment such as hydrothermal vents.
The discovery that RNA appears to be the agent of peptide-bond synthesis in the modern ribosome (Guthrie 1992, Pace 1992, Noller et. al. 1992) and the capacity of modified ribozymes to act as amino-acyl esterases (Picarilli et.al. 1992), the first step of ribosomal action in protein synthesis, establish RNA has the potential to act as synthetase as well as transfer, messenger and ribosomal functions. This gives RNA the capacity to act on its own to catalyse both its own replication and the ordered polymerization of proteins. Simpler model systems have also been advanced of the stereospecific capacity of D-nucleotides to act as a catalyst of L-amino acid polymerization (Lacey et. al. 1990). These results enable RNA to be the key prebiotic molecule generating ordered polynucleotide and polypeptide structures.
Szostak and Wilson (1996, Wilson and Szostak 1995) have evolved ribozymes capable of a broad class of catalytic reactions. The catalysis of previous ribozymes tended to involve only the molecules' sugar-phosphate "backbone," but these could also promote the formation of peptide bonds (which link amino acids together to form proteins) and between carbon and nitrogen. (Horgan). David Bartel a former member of Szostak's team, has evolved RNAs that are as efficient as some modern protein enzymes. The problem with most ribozymes is that they are as likely to snip an RNA molecule apart as stitch one together which makes copying a molecule fifty nucleotides long (the minimum size necessary to catalyse a chemical reaction) difficult or impossible. Bartel's new ribozymes, on the other hand, can stitch small pieces of RNA together without breaking larger molecules apart. These ribozymes use high-energy tri-phosphate bonds similar to ATP as their fuel, speeding the reaction up several million-fold. "We've got ribozymes doing the right kind of chemistry to copy long molecules" says Szostak "We haven't achieved self-replication from single nucleotides yet, but it is definitely within sight" (Cohen).

Zhang and Cech have reported a step towards the goal of linking amino-acids. They isolated RNAs that could efficiently link specific amino acids together (Zhang and Cech 1997). These pseudo-ribosomes were selected from a random pool of 1015 synthetic RNAs. They then elicited a trans-acting by coupling one of the amino acids to a short RNA with complementary sequence to the ribozyme achieving a ribozyme which whould join a ribosynthetase-amino acid to form a peptide bond with another thus relicating even more closely ribosomal function. They also found that a small region of many of the RNAs they selected was 70 per cent identical to some regions of the ribosomal RNA. "We not only copied ribosome function, we seemed to have recapitulated its evolution," says Cech. The two researchers then removed or mutated these sequences in the synthetic RNAs (Zhang and Cech 1998) any change to this region cut the activity of the RNA by a factor of between 20 and 600. This suggests this region in both the modern ribosome and the synthetic RNA may have the same role in the fusion reaction, such as holding the amino acids in the correct position and that they may have converged on the same molecular solution.

Fig 25b: (a, b) Three component system of RNAs acting as a ribozyme to form a catalytic netwok (Vaidya et al. 2012).
(c,d) Self-sustained replication of an RNA system (Lincoln and Joyce (2009).
Several groups have reported success in forming model systems of RNA ligases which can replicate themselves from smaller oligo-RNAs, by contrast with RNA synthesis from monomers, which is currently not self-sustaining and possible only under externally controlled conditions. Lincoln and Joyce (2009) demonstrated self-sustained replication of an RNA enzyme which could spontaneously ligate oligomer precursors and Robertson and Joyce (2014) followed this up with a more efficient system replicating in a cycle of under 5 mins. Vaidya et al. (2012) have shown that mixtures of RNA fragments of an Azoarcus group I intron ribozyme can self-assemble into self-replicating ribozymes that spontaneously form cooperative catalytic cycles and networks. A recent breakthrough was made by recombining traits evolved separately in different ribozyme lineages in combination with in-vitro evolution and engineering producing an RNA polymerase ribozyme (tC19Z in fig 25), capable of synthesizing RNAs of up to 95 nucleotides in length, that was able to synthesize a spectrum of RNA sequences, including the accurate synthesis of an enzymatically active RNA, a hammerhead endonuclease ribozyme (Wochner et al. 2011).
Viroids which parasitize higher plants and possibly cyanobacteria also have several markers of molecules from the RNA era, including ribozyme activity, lack of protein coding genes and self-priming re-lication from circular intermediates. Introns, the non-coding regions that punctuate functional regions in eucaryotes, some of which have self-splicing ribozyme actiity, also show attributes consistent with the RNA era.
Several of these approaches suggest that autocatalytic networks of small molecules with oligomers of ribonucleotides and peptides of various sizes may have produced systems that can self-organize to precipitate a milieu in which cooperative RNAs can replicate and evolve. Several groups have used a combination of chemical and computational approaches to try to establish the viability of such autocatalytic networks (Ashkenasy et al. 2004, Hordijk 2015).
Another older hypothesis is that life may have begun as a molecular hybrid, PNA or peptide nucleic acid. PNA has a similar structure to RNA except for having a peptide backbone based on prebiotically abundant glycine and can co-instruct complementary RNA sequences and vice versa (Bohler, Nielsen and Orgel). The bases of PNA are joined together with peptide links like those in proteins which may not present the instabilities which sugars may have faced on the early earth. Matthew Levy and his colleagues (Nelson, Levy and Miller) persuaded up to 78 per cent of plausible prebiotic chemicals to transform into PNA backbone subunits amino-ethyl glycine or AEG. The acetic acid derivatives of the bases A, G U and C can likewise be generated from prebitotic reagents including NH4CN with glycine and cyanoglyceraldehyde. AEG units link up readily at 100 deg C, which may have been common temperature four billion years ago when our planet was rich in volcanic activity. PNA is clearly an alternative route to establishing the RNA era which also has a good cosmological foundation. However researchers concede that there is no evidence such alternative molecules have existed in Earth's history "You don't see a smoking gun," says Gerald Joyce, of the Scripps Research Institute (Marshall M 2011).
Fig 25b2: 2-amino-adenine can also be used as a building block for genetic replication.
A further issue is whether the standard A, G, C and U were to optimal nucleotides. Some phage viruses have adapted to utilise 2-amino-adenine, which like the GC bond has a triple H-bond, however this appears to be a subsequent evolutionary step to bypass the usual bacterial resistance measures against viral invasion, bu tit does pose the question as to whether other combinations of heterocyclic bases could become the foundation of neuceotide replciation and the eventual genetic code (Saey 2021).
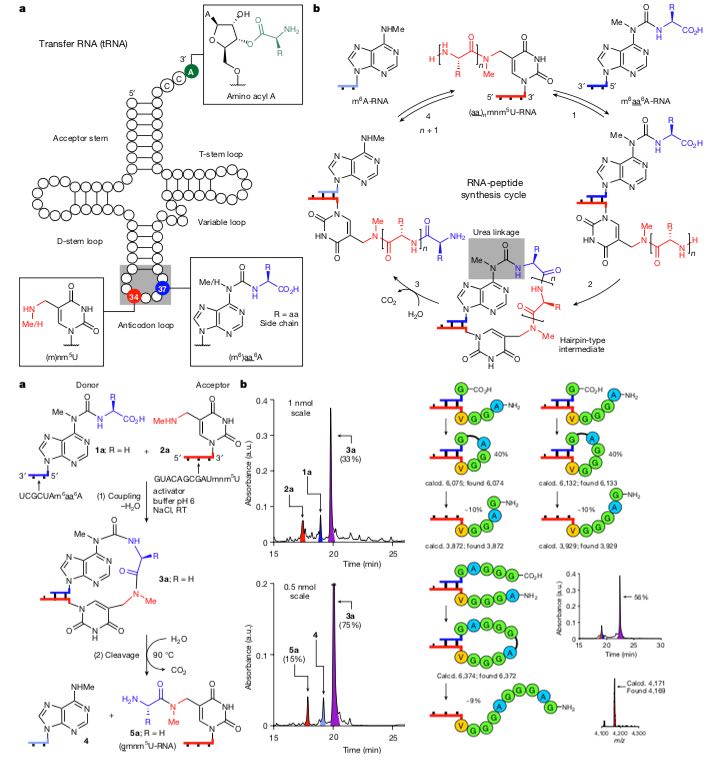
Fig 83b: A primitive precursor has been discovered (Müller et al. 2022) to the dependence of RNA-based protein on the ribosome (lower left) involving both two large rRNAs, and small t-RNAs specific for each amino acid which have to be coupled to their amino-acids by protein synthetases. Modified bases, still found on transfer RNAs (top-left) have been found to be able to induce formation of oligomeric chains of peptides (lower right) by a cyclic process involving base pair binding between small RNAs carrying these modified bases (top right), confirmed by the chromatography rests (lower centre). This removes an obstruction to spontaneous abiogenesis, in which the ribosomal machinery could have been regarded as an irreducibly complex system requiring intelligent design and replaces it with a fecund population of hybrid RNA-peptide molecules
Müller et al. (2022) provide another ground-breaking discovery, showing that the modified RNA bases still present in transfer RNAs can when two RNAs hydrogen bond together can promote the polymerisation of amino-acids providing a precursor route to the translation apparatus that later emerged in the ribosome. These modified bases, attached to short RNAs like t-RNAs can co-synthesise peptide chains, forming diverse hybrid RNA-peptides with all kinds of catalytic functions no one had thought of.
Here we have a demonstration of a completely new insight which has already been experimentally confirmed in the liquid chromatography assays in the figure and is thus feasible with the modified bases still existing in t-RNAs, which is surprising in itself. Originally there would have been more. This in one fell swoop completely demolishes any irreducible complexity argument for the ribosome. It transforms the paradigm of abiogenesis, because it means that confining RNAs to be pristine and proteins to be pristine is a misconception that has arisen because genetically coded translation later refined it to be like that. The underlying process is highly fecund as we should have expected, because amino acids are cosmologically abundant more so than nucleotide bases and have much more diverse catalytic diversity, so hybrid RNA-polypeptide molecules have vastly more catalytic and complexity capacity to bootstrap abiogenesis than even the most "hard core" prebiotic scientists conceived of. The picture that emerges is a new generation of fecund molecules with an RNA backbone and active amino-acid moieties able to do all sorts of things we had not thought of at all.

Proto-ribosome, consisting of RNA molecules comprising only of some 120 nucleotides, about 60 for each of its two semi-symmetrical components, which accounts for less than 5 percent of the modern ribosome's dimensions: some 4,500 nucleotides in bacteria and nearly 6,000 in humans. "Peptide bond formation is the most vital activity in any cell, and we've shown that it can take place within a protoribosome" (Bose et al. 2022).
Bose et al. (2022), fig 56 have taken this a step further, discovering a semi-symmetrical proto-ribosome capable of forming peptides with only 5% of the ribosomal rRNA. These discoveries in one fell swoop demolish any irreducible complexity argument for the ribosome, transforming the paradigm of abiogenesis.
Fig 25c: Small and large rRNA subunits of the eubacteia Thermus thermophilus and the archaeon Haloarcula marismortui.
RNA orange and yellow, protein blue and active site green. (Wikipedia Ribosome) Click HERE to see the images rotating.
A central focus of the origin of life as we know it is the formation of the ribosome, which enables translation of the genetic code into functioning proteins by assembling the amino-acids unit by unit. The core of the ribosome is RNA, with the additional proteins having only a supporting function as illustrated in the fig 25c. From one point fo view this lends strong support to the idea of the RNA world because the core of the machinery to assemble proteins consists of RNA. However some researchers have suggested that the specificity of RNA operating on its own is too low to support coding of functional enzymes. They suggest instead an era in which the primitive ribosome began producing a relatively random collection of co-polymers based on the diversity of primitive syntheses and only after further refining did natural selection kick in to provide an RNA-protein core for life (Holmes 2015).
A specfic model of the evolution of the ribosome envisages that the smaller subunit which inds to and moves along the mRNA began first as an RNA helicase which was essential to avoid the RNA era ending in non-replicating double stranded hairpins (Zenkin 2012). The helicase moved down double stranded regions, regenerating ribozyme functionlaity. Later this became associated with the larger subunit iniially low-specificity polypeptides e.g. with a polar vs non-polar code determining conformation energetics in the aqueous medium. Amino-acid couplings to tRNAs may have initialy been established through ribozymes. Only once the primitve genetic code emerged did translation proper begin.
UNIVERSAL STABILITY STRUCTURES IN MOLECULAR BIOLOGY
The previous discussion of the RNA era can unravel a double-bind that is central to biogenesis - how did the core biochemical pathways become generated? The traditional viewpoint is that they were successively created starting from a simple chemical-feeding heterotroph, through mutational evolution, building one-by-one the protein components necessary to make a working whole. This however does not explain how integrated systems such as electron transport and the citric acid cycle could have functioned at all with only a vestigial complement of enzymes.
This suggests that many of the major features of molecular biology are generic structures which can come into existence under suitable conditions, through bifurcation, independently of the emergence of genetic RNA, and that these were subsequently captured by genetic takeover as genetic complexity permitted. Such generic structures include the polymeric structure of proteins and nucleic acids, nucleotide coenzymes, bilayer membrane structure and the topological closure of the cell, ion transport and membrane excitability, membrane-bound electron transport, glycolysis and the citric acid cycle.
Such a perspective has far-reaching consequences for molecular biology in cosmological terms, for while the details of mutational evolution will be unique to each environment, the major features underlying biology could be universal.
(a) Translation and the Genetic Code. According to the genetic takeover hypothesis, evolution of RNA captured existing stability structures in the prebiotic medium. The most central of these is clearly the use of proteins as coded enzyme catalysts. Such a process could only have occurred in an environment in which RNAs coexisted with amino-acids and in which a very small additional genetic advantage could capitalize on simple coding of existing structures to good effect.
A variety of amino acids and oligopeptides are common products of prebiotic syntheses. The polymerization of amino acids and the development of peptide backbones with cyanide side chains from the linear HCN oligomer fig 16, provide alternative routes to oligopeptide structure. A natural propensity for -NH2 and -CO2H moieties as basic and acidic groups arises directly from the electronegativity bifurcation.
The discovery that ribosomal, synthetase, messenger and transfer functions of protein synthesis can all in principle be carried out by RNAs alone leads to a natural interpretation of the development of the genetic code from a protein-free translation system. The major partitions of the genetic code have structural features consistent with an origin in underlying chemical bifurcations.


Fig 26: Left: Birfucation model of the genetic code (King 1982). Right: Functional model (Knight, Freeland & Landweber. 1999).
Although there are many other similar and varying invoked scenarios, including functional associations, as illustrated in fig 26 right (Knight, Freeland and Landweber 1999), a model generated on the basis two overlapping doublet codes one working on the first two bases and the other on the second two, which became merged in the triplet code explaining some features of the code (Wu et al. 2005), and compositional analyses suggest that Gly, Ala, Asp, Asn, and Thr are among the most ancient amino acids in the code, while Glu, Gln, Phe, Tyr, Trp, Cys, and Ser are later additions (Fournier & Alm 2015), we will outline in detail the fundamental bifurcation sequence of fig 26 left, which also coincides with early protein evolution being based on hydrophilic vs hydrophobic affinities, as follows (King 1982):
1 Polarity bifurcation: There is a major bifurcation in polarity between amino acids with anticodons having centre bases U & A. Uracil is correspondingly more hydrophilic than adenine, as reflected in their dominant split in hydrophobicity A(3.86)>G(2.3)>C(1.5)>U(1.45) and water solubilities A=1/1086, U=1/280. This leads to the idea that the polarity bifurcation was a principal symmetry-breaking factor in the origin of the nucleic acid code.
The discovery or L-aspartic and glutamic acids on Rosetta suggests a primal code based on polar aspartic and glutamic acids counterposed to non-chiral glycine and some other raecemic non-polar amino acids such as alanine giving rise to polypeptides with specific micelle structures.
2 Abundance and GC: The initial base G also codes the most abundant amino acids, consistent with a GXY code starting with GAY=polar (anticodon U), GUY=non-polar (anticodon A) providing binding strength of GC and frame shift suppression (Y=pyrimidine).
3 Four-fold code: Extending to include GGY, GCY, provides a fourfold specificity for polar (Asp/Glu), non-polar (Val and larger), along with Gly, and Ala as most abundant.
4 Eight- and Twelve- fold codes: This could have then doubled to and 8-word code by including CAY, CUY, CGY, and CCY coding for non-polar and basic groups, and then a similar series based on AAY, AUY, AGY, and AC.Y Wong (1975, 2005) originally noted a correspondence between the first codon base and biosynthetic pathways in primitive organisms such as sulphur bacteria with Pro, Arg, Gln Leu, His derived from Glu and having first codon base C and Ser, Thr, Ile, Asn, Met, Lys being derived from Asp having first codon base A (Knight, Freeland and Landweber 1999). OH- and SH-containing amino acids also form a single additional block (UA)(GC)Y, suggesting a third bifurcation for H-bonding, with UAY reading stop. Notably these is significant stereospecific affinity between certain amino acids such as Ile and Arg and their codons (ibid).
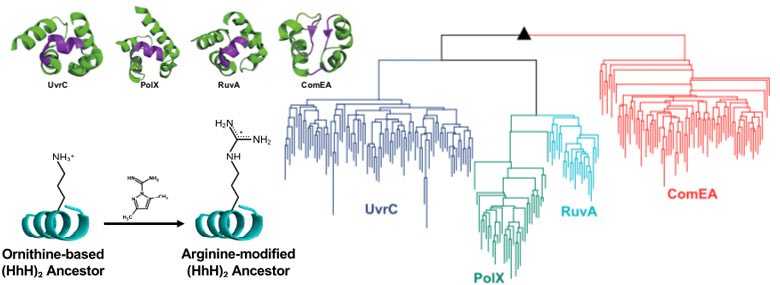
Fig 26a; Pathway to nucleotide-binding polypeptides utilizing abiotically guanidinated ornithine.
A key step in this process could have been the primordial role of ornithine which is a basic amino acid, but unlike arginine which lacks a prebiotic synthesis does occur in Miller-Urey syntheses. The role of ornithine is then proposed as a precursor of arginine and lysine, basic amino acids critical in nucleotide-binding proteins. Dan Tawfik and his team (Longo L et al. 2020) have discovered that an evolutionary history of such proteins -- UvrC, PolX, RuvA,mand ComEA -- lead back to a putative ancestor consisting of an ornithine-containing polypeptide. They have also discovered an abiotic synthesis route based on guanidination the same chemical process driven by enzymes to synthesise arginine in living cells, that could convert the ornithine side-chains to arginine, inducing prebiotic polypeptides with nucleotide-binding properties.


Fig 26b: Recent model of peptide-RNA interaction at the formation of the genetic code. Left: Hydrophobicities of amino acid side-chains at 25oC and 100oC, colored according to the middle base of the corresponding codon. Hydrophobic residues, near the top, tend to be associated with pyrimidines [red and purple], whereas hydrophilic residues, near the bottom, tend to be associated with purines [blue and black] (Wolfenden et al. 2015). Right: Orthogonal transfer free energies predicted by the most important bases in tRNA acceptor stems and anticodons. The acceptor stem distinguishes between β-branched and nonbranched side-chains but fails to identify aromatic, positively charged, or amide- containing side-chains correctly. The anticodon identifies these correctly but fails to distinguish β-branched from non-branched side-chains. Bases of both the acceptor stem and anticodon identify carboxylate side-chains correctly, suggesting that such side-chains may have played an unusually important role in both early and late stages of protein evolution. Acceptor stem coding may have conferred a selective advantage by distinguishing smaller from larger amino acids and identifying β-branched, aliphatic, and carboxylate side-chains. Carter & Wolfenden (2015, 2016) suggest that genetic coding evolved in distinct stages. Initial discrimination on the basis of size may have allowed coding by tRNA acceptor stems to ensure that the earliest peptides were β-structures with alternating large and small side-chains, to interact with RNA, and only later encoded globular conformations with greater catalytic activity. The earliest peptides may have included the unstructured peptide tails that stabilize ribosomal RNA. Carter and Wills (2017, Wills & Carter 2017) have also tried to demonstrate that these features imply emergent translation is ancestral and that replication in an RNA-only era presents insurmountable problems.
A pivotal idea that is central to the establishment and evolution of the genetic code is the feedback loop through t-RNA synthases that is essential for the ribosome to be able to encode proteins via their t-RNAs. The synthases come in two classes, which have completely different protein structures and also couple onto the t-RNA in different ways, involving both the 2OH vs 3OH attachment sites in the terminal adenosine and the major or minor groove involved in the approach. The Rodin-Ohno (1995) hypothesis that these two classes originated from complementary sense and anti-sense transcripts of the same gene has received experimental support from studies (Martinez-Rodriguez et al. 2015, Carter 2017) showing that when the enzymes are pruned down to their evolutionary core they remain catalytic by several order of magnitude. This provides convincing evidence for a primal origina from a single gene able to catalyse distinct classes of amino acid by distinct processes. These may have both initially been involved together facilitating overall coupling (de Pouplana 2020), before adopting complementary roles as their enficiency increased. Carter & Wills (2018, Wills & Carter 2018) have established routes of viable establishement of the underlying feedback loops enabling the emergence of amino-acid selectivity.

Fig 26b2: (a) Possible evolution of t-RNA synthases from a complementary pair of sense and antisense enzymes (de Pouplana(2020). (b) The Rodin-Ohno (1995) hypothesis of paired sense-antisense transcripts leading to class 1 & 2 synthases. (c, e) The complementary structures of the two classes and their amino acid assignments (Kaiser et al. 2018). (d) Overall structures (Wikipedia) (f) The complementary enzymes retain catalytic activity when reduced to a 49 amino acid evolutionary core based on complementary DNA strands (Martinez-Rodriguez et al. 2015). (g) Evolutionary processes driving formation of the genetic code (Carter 2017). (h) tRNA acceptor-stem bases considered in the analysis of aaRS groove recognition (Carter & Wills 2018).

Fig 26b3: AARS accretion model. Branching off from the central black-and-white ancestral lineages into extant proteins could have occurred at any time, and hence arrows do not denote the passage of time, but rather evolutionary relationships. A phylogenetic reconstruction of extant AARS genes, enhanced by analysing modular acquisitions, reveals six AARS with distinct bacterial, archaeal, eukaryotic, or organellar clades, resulting in a total of 36 families of AARS catalytic domains. Small structural modules that differentiate one AARS family from another played pivotal roles in discriminating between amino acid side chains, thereby expanding the genetic code and refining its precision. The resulting model shows a tendency for less elaborate enzymes, with simpler catalytic domains, to activate amino acids that were not synthesised until later in the evolution of the code. The most probable evolutionary route for an emergent amino acid type to establish a place in the code was by recruiting older, less specific AARS, rather than adapting contemporary lineages (Douglas, Bouckaert, Carter & Wills (2023).
5 Evolutionary takeover: From this point evolutionary selection begins to optimize the bifurcations caused by stereospecificity and the growth of these interactions into synthesis pathways, based on error minimization and the incorporation of the last of the amino acids. Later assignments such as Trp are consistent with evolutionary adaptions. Brooks et al. (2002) have found that the amino acids used in sections of genes common to life which are believed to originate with the last universal common ancestor show amino acid distributions reflecting the relative abundance of such amino acids in primitive synthesis indicating that the first translational genes used the amino acids which were spontaneously available.
Many of the amino acids also have essential molecular interactions in the metabolism which could have influenced their incorporation into the genetic code. Glutamate and glutamine are essential components in nitrogen assimilation, serine and glycine are essential in single-carbon metabolism, cysteine and methionine are central to sulfur uptake and metabolism. Many amino acids are also involved in small molecule/cofactor synthesis and RNA modification (Fournier et al. 2011).
Freeland and Hurst (1998), have shown that strong selective pressures must have acted on the code during its evolution. Hurst found that single-letter changes to a codon, inserting the wrong amino acid into a protein, tended to specify amino acids that were very similar chemically to the correct ones, minimising the impact on the protein. Freeland then reasoned that the code should minimise chemical differences most between the correct and incorrect amino acid at the third base in the codon since translation misreads this base 10 times as often as the second. In an analysis that gave extra mathematical weight to the vulnerable sites most likely to be mistranslated, Freeland showed that no more than one in a million random codes was better at reducing the impact of errors than the natural code. The possibility of evolutionarty change in the code is affirmed by both mitochondrial and nuclear variants (Knight, Freeland and Landweber 1999).
Following on from this Freeland et. al. (2000) have analysed other work showing that more optimal global solutions do exist to propose that stereochemical and synthesis path constraints fixated the code early on into one which was later evolutionarily optimized on error minimization constraints, the modern code being optimal under these constraining conditions. This analysis gives strong weight to the idea that the form of the code is derived from chemical, historical and selective factors rather than being a frozen accident which happened to the predecessors of the last common ancestor of living cell lines.


Fig 26c: (Left): Trees for Ser, Pro and Thr aaRSs each show both (a) a common ancestor and (b) rare and universal forms consistent with horizontal transfer from a sister species of LUCA. (Right): Trees for Tyr and Trp likewise show (a) a common ancestor and a more recent emergence of the Trp aaRS, as its tree follows existing evolutionary relationships of TACK as the archaeal group closest to eucaryotes, while that of related Tyr is more complex and indicates an origin before LUCA.
Fournier and coworkers (2011, 2015) have investigated in detail the evolutionary pathways leading to the aminoacyl-tRNA synthetases (aaRS), coupling an amino acid to its respective t-RNA. Unlike the rest of the translation machinery, such as rRNAs, where the evolutionary tree has strong vertical tree alignment, aaRS have undergone numerous ancient horizontal gene transfers (Wolf et al. 1999), with several independent events detected between domains, and some possibly involving lineages diverging before the time of LUCA, the last universal common ancestor of existing life, as sequence analysis of their putative precursor indicates it is older than LUCA. On the other hand, many core protein families with similar functions are paralogs, generated by gene duplication, suggesting a common ancestor with a similar function. These relationships are especially apparent between several sets of aaRS proteins (TyrRS/TrpRS, IleRS/ ValRS, GluRS/GlnRS, AspRS/AsnRS, CysRS/MetRS).
Sequence reconstructions of the paralog ancestors of isoleucyl- and valyl- RS provide evidence that at least for this divergence, the genetic code did not co-evolve with the aaRSs. Rather, both amino acids were already part of the genetic code before their aaRSs diverged from a common ancestor, with ambiguous affinity. This picture is consistent with a primordial synthesase activity based on ribozymes whose function became transferred over time to protein synthesases - encoded polypeptide biosynthesis and tRNA almost certainly pre-existed, with the earliest protein synthesis emerging in a world where functions of modern proteins were already being performed by another - e.g. ribozymal - system (Xiao & Yu 2007, Knight et al. 1999).
It is fairly obvious from the genetic code (fig 26) that tryptophan appears to be one of the last amino acids to become incorporated in the genetic code, with its codon 'belatedly' inserted into the stop area, with the possible exception of seleno-cysteine, which requires additional modifications on the fly. Comparison of the evolutionary trees of tyrosine and tryptophan, which share a common precursor, demonstrates (a) that horizontal transfer(s) have occurred, possibly from a sister organism of LUCA, creating rare forms not general to all branches of LUCA, posing a similar situation to the introgression of Neanderthal DNA into Homo sapiens, and (b) that the tryptophan synthesase evolved later than that of tyrosine, after the code was already in place, as the evolutionary diversification follows existing LUCA relationships for tryptophan but not for the older tyrosine. Ancestral sequence reconstructions of the pre-LUCA paralog ancestor of TyrRS and TrpRS have several sites containing Tyr, yet a complete absence of sites containing Trp. This is consistent with the paralog ancestor being specific for the utilization of Tyr, with Trp being a subsequent addition to the genetic code. The presence of absence of tryptophan in a core protein can therefore be used as an estimate of how ancient it is.
 Fig 27: The continued ubiquitous central function of nucleotide coenzymes in central metabolic pathways attests to them being evolutionary fossils from an era in which nucleotides functioned both as catalysts and transporters as well as informational replicators. ATP besides being the universal energy activator forms a component of many cofactos. GTP likewise has a central role in the energy source for translation steps. U and C ribonucleotides have specific roles as transporters.
Fig 27: The continued ubiquitous central function of nucleotide coenzymes in central metabolic pathways attests to them being evolutionary fossils from an era in which nucleotides functioned both as catalysts and transporters as well as informational replicators. ATP besides being the universal energy activator forms a component of many cofactos. GTP likewise has a central role in the energy source for translation steps. U and C ribonucleotides have specific roles as transporters.
(b) Nucleotides and the Nucleotide Coenzymes. The nucleotide co-enzymes are widely regarded as ancient molecular fossils retained from the RNA-era. In addition to the key role of ADP and ATP as energy currency in the bio-metabolism, GTP is used in protein synthesis, and the nucleotides UDP and CDP are carriers of glucose and choline and other membrane components. Model prebiotic reactions have successfully coupled UDP and CDP to glucose and choline (Mar et. al. 1986). Both NAD, and FAD function as carriers of redox energy. Coenzyme A consists of adenosine coupled to pantothenic acid and functions as a carrier of acyl and other groups via the terminal SH bond (Reanney 1977). Cobalamin - vitamin B12 - also illustrates how a di-nucleotide can bind a metallic porphyrin-type corrin ring. Eschenmoser (1988) has also discovered a plausible prebiotic pathway generating the more complex B12 molecule which involves two nucleotides and a Co-porphyrin. In organisms which cannot make the purine 5,6-dimethyl-benzimidazole both the nucleotides are adenine. As B12 is essential for methionine synthesis, it antedates the genetic code.
 Fig 28: (a) Di-phosphorylation of sugars leads to glycolysis through interaction of charged phosphates.
Fig 28: (a) Di-phosphorylation of sugars leads to glycolysis through interaction of charged phosphates.
(b) Generic examples of group transfer in the tricarboxylic acid cycle.
(c) Glycolysis and Phosphorylation
Glycolysis forms a bridge between six and three carbon sugars, reversing the structural pathway from H2CO, glycoaldehyde and glycderaldehyde to cyclic sugars, fig 17(b). Glycolysis is made energetically possible by phosphorylation, and releases high energy phosphate capable of driving other phosphorylations (Hermes-Lima and Vieyra 1989), fig 28(a). It is notable that glycolytic di-phosphorylation of fructose is homologous with the role of phosphate in nucleotide formation and oligomerization. The high phosphate environment leading to RNAs would then naturally lead to similar phosphorylation of other sugars, and release of the high-energy phosphate bond through cleavage of the sugar. Mineral catalysis associated with phosphate gives the glycolytic pathway a natural basis for lysis of sugars as a dissipative structure. Biological UDP-glucose coupling is consistent with nucleotide-dependent glycolysis in the RNA era.

Fig 29: Keller et al.'s reaction pathways in primitve ocean model and biochemical metabolism compared
The reaction sequences of central metabolism, glycolysis and the pentose phosphate pathway provide essential precursors for nucleic acids, amino acids and lipids. Keller et al. (2014) reconstructed potential scenarios for oceans of the prebiotic Archean based on the composition of early sediments. The resultant reaction milieu catalyses the interconversion of metabolites that in modern organisms constitute glycolysis and the pentose phosphate pathway. The 29 observed reactions include the formation and/or interconversion of glucose, pyruvate, the nucleic acid precursor ribose-5-phosphate and the amino acid precursor erythrose-4-phosphate, antedating reactions sequences similar to that used by the metabolic pathways. The Archean ocean mimetic increased the stability of the phosphorylated intermediates and accelerated the rate of intermediate reactions and pyruvate production. The reactions were particularly sensitive to Fe2+, which is understood to have had high concentrations in the Archean oceans. Reaction sequences that constitute central carbon metabolism could thus date back to the prebiotic world.
(d) The Membrane, Excitability and Ion Transport :
All life as we know it is dependent on maintaining a distinct internal micro-environment as an open far-from-equilibrium thermodynamic system (Glansdorff and Prigogine), through the topological closure of the cell. Viruses for example all depend on cellular life. The structure of the bilayer membrane is a direct consequence of the polarity bifurcation. The formation of amphophilic lipid-like molecules, joining a linear non-polar hydrocarbon section to an ionic or H-bonding polar terminal, leaves 2 degrees of freedom for layer formation. Backing of the non-polar moeties to one another, fig 30, completes the bilayer. Cell structure can then arise directly from budding of the bilayer, as illustrated in budding in several types of prebiotic reaction medium. Microcellular structures are abundant in many origin of life syntheses, fig 16. The use of CDP associated with choline, inisotol & lipids in membrane construction is consistent with membrane formation in the RNA era. The structure of typical biological lipids such as phosphatidyl choline display a modular structure similar to ATP, consisting of fatty acid, glycerol, and substituted amine again linked by dehydration and involving phosphate, fig 31(e).
 Fig 30: The extremely ancient origin of the rhodopsin family of heptahelical receptors can be seen from the ultra-primitive photosynthesis in Halobacteria, a halophilic member of the archaea, which lacks any form of electron transport, relying on direct coupling between photo-stimulated chemiosmotic H+
pumping and H+ generated ATP formation, based on bacteriorhodopsin, which is heptahelical, uses retinal and shares distant sequence homology with vertebrate rhodopsin. The ATP-synthase which has a rotary motor may have evolved from two separate proteins, with the rotary engine arising from a primitive helicase.
Fig 30: The extremely ancient origin of the rhodopsin family of heptahelical receptors can be seen from the ultra-primitive photosynthesis in Halobacteria, a halophilic member of the archaea, which lacks any form of electron transport, relying on direct coupling between photo-stimulated chemiosmotic H+
pumping and H+ generated ATP formation, based on bacteriorhodopsin, which is heptahelical, uses retinal and shares distant sequence homology with vertebrate rhodopsin. The ATP-synthase which has a rotary motor may have evolved from two separate proteins, with the rotary engine arising from a primitive helicase.
The existence of the membrane as a non-polar structure leads to segregation into ionic and non-polar reaction phases. Ion transport is essential in maintaining the concentration gradients that distinguish the cytoplasm from the external environment and thus must develop in the earliest cellular systems (MacElroy et. al. 1989). Ion transport is a source of significant electronic effects, because the membrane under polarization is piezo-electric and is capable of excitation in the presence of suitable ions. Model systems using the simple 19 unit oligopeptide Na-ionopore alamethicin and artificial membranes display action potentials (Mueller and Rudin 1968). Similar results have been reported for microcells produced by prebiotic techniques containing light irradiated chromophores (Przybylski and Fox 1986), demonstrating that such effects are fundamental to the quantum architecture of lipid membranes (King 1990). Four groups of non-polypeptide neurotransmitters: acetyl-choline, catecholamines, serotonin and histamine are amines, the latter three being derived from amino acids tyrosine, tryptophan and histidine by decarboxylation. Two others are amino acids and thus also contain amine groups. Notably alamethicin also has glutamine amides located in the core of the pore (Fox & Richards 1982). The catecholamines are linked to indoles such as serotonin by a prebiotic pathway, fig 31(c).
 Fig 31: (a) Phosphate energy in glycolytic splitting (b) Tricarboxylic acid transformations
Fig 31: (a) Phosphate energy in glycolytic splitting (b) Tricarboxylic acid transformations
(f) The Tricarboxylic Acid Cycle :
forms a pool of multiply carboxylated molecules which carry CO2 in various states of energy, and result in reducing energy via nucleotide coenzymes NAD and FAD, which coupled with the use coenzyme A provide a basis for the tricarboxylic acid cycle in the RNA era. This could have existed as a limit cycle of di- and tri-carboxylated molecules acting both as an acceptor of acetate (a carbohydrate-equivalent i.e. (H2CO)2) and as an emitter of molecular CO2 and reducing H, thus bifurcating carbohydrate level redox potential into reduced and oxidized components.
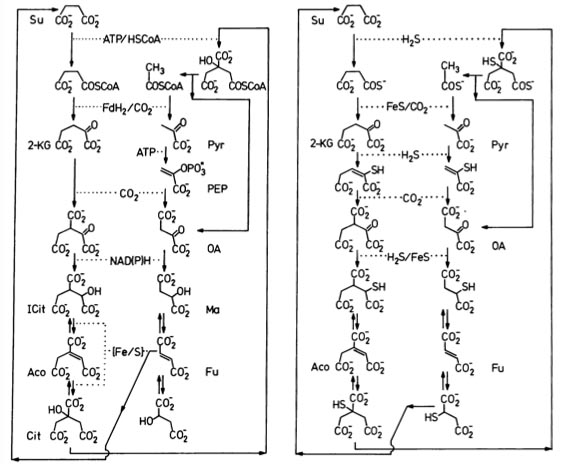 Fg 31b: Hypothetical primitive tricarboxyllic acid cycle (right) exploiting sulphur bonds which could couple to sulphur-based pyrite energetics (Wachterhauser 1990).
Fg 31b: Hypothetical primitive tricarboxyllic acid cycle (right) exploiting sulphur bonds which could couple to sulphur-based pyrite energetics (Wachterhauser 1990).
The linkage to nucleotide coenzymes such as NAD would have served to create a bifurcation of redox potential in the molecular milieu contributing to the diversity of reacting species. The cycle may have been hypercyclic (Eigen et. al. 1981) or based on sulphur energetics (Wachterhauser 1990) in a manner which might also allow a series of coupled short cycles, or could even have been a network, consisting of a population of molecules undergoing various generic transformations with net inflow of carboxylic acids and net emission of CO2 and transfer of H, due to generic transformations as illustrated in fig 33(b). Isomerization would have been catalysed by Fe2+. Several steps may have been driven by sunlight photolysis (Waddell et. al. 1989).
The probability that the the central structures of molecular biology existed in the RNA era is consistent with their being chemical stability structures utilized by catalytic RNAs. The small genomes during the RNA era and limited catalytic capacity of RNAs by comparison with protein makes it likely that the emerging RNA-based system had to capitalize on existing chemical stability structures becase it lacked enzyme-based biosynthetic pathways. Genetic takeover also places these stability structures in a category determined by the cosmological milieu, thus giving evolutionary biology a cosmological foundation.

Fig 31c: The tricaboxylic acid cycle (TCA) is one of the core metabolic pathways in many present-day organisms and is also reversible leading to the reductive TCA. Muchowska et al. (2019) report that nine (red) of the eleven intermediates in the TCA cycle (left) are also formed in a complex network of reactions (right) that is established when glyoxylate and pyruvate are combined in water with ferrous iron (Fe2+). The authors propose that this might have formed a non-biological framework for metabolic pathways when life emerged on early Earth.
In an analytical breakthrough, Muchowska et al. (2019), reporting the complex network of carboxylic reactions above which synthesize 9 of the 11 intermediates in the tricoarboxulic acid cycle, also show that, in the presence of hydroxylamine (NH2OH) and metallic iron, their chemical network can be extended to include the formation of four kinds of amino acid, the building blocks of proteins. Both hydroxylamine and metallic iron could have been available on early Earth: hydroxylamine would probably have formed as a result of the rich, abiotic nitrogen chemistry that is known to have occurred early in the planet's existence2, whereas metallic iron is abundant in certain meteorites that peppered our planet..
 Fig 32: (a) NAD structure permits linkage of other energies to a redox bifurcation. (b) H+ and e- transport linked by H2 in membrane due to insolubility of e- and solubility of H+. (c) Prebiotic link between catecholamines and indole via quinone-type photoreduction. (d) Hypothetical form of primitive electron transport as a non-equilibrium limit cycle. (e) Acetyl-choline and phosphatidyl choline compared. Phosphatidyl choline lipid stacks tail to tail as shown in the clothes pegs (b).
Fig 32: (a) NAD structure permits linkage of other energies to a redox bifurcation. (b) H+ and e- transport linked by H2 in membrane due to insolubility of e- and solubility of H+. (c) Prebiotic link between catecholamines and indole via quinone-type photoreduction. (d) Hypothetical form of primitive electron transport as a non-equilibrium limit cycle. (e) Acetyl-choline and phosphatidyl choline compared. Phosphatidyl choline lipid stacks tail to tail as shown in the clothes pegs (b).(e) Electron Transport
The fact that the proton is soluble in water to form the hydrogen ion, but the electron is not, unless attached to another group such as a protein, causes a physical linkage to exist between the polarity bifurcation and the charge bifurcations associated with electron and proton transfer, fig 31(b) mediated by H transport through quinone reduction, (c). Despite the complexity of modern electron transport in photosynthesis and respiration, there is considerable evidence that membrane electrochemistry could have arisen before translation could produce coded enzymes. Firstly there is a consistent basis for the existence of many of the components of electron transport during the RNA era, since the nucleotide coenzymes NAD, FAD, a nucleotide-bound Mg & Fe-porphyrin ring similar to B12, a cysteine-bound FeS group (Hall et. al. 1974), possibly based on glutathione (g-glutamyl-cysteinyl-glycine) and quinones provide all the key components of electron transport in an RNA dependent but protein-free form, fig 31(d) (King 1990).  Both porphyrins and quinones have obvious prebiotic syntheses and the primal role of nucleotide coenzymes has already been discussed. Secondly, membrane structure and the solubility differences between the electron and proton guarantee a link between electron and hydrogen ion transport fundamental to quantum symmetry-breaking. Electron transfer does not require the complex coded active sites required to catalyse specific molecular transformations. Model systems using Fe-porphyrins and imidazole can couple oxidative electron transport to phosphorylation (Brinigar et. al. 1966) and photo activated Mg-porphyrin to phosphate link (Goncharova and Goldfelt 1990, Lozovaya et. al. 1990).
Both porphyrins and quinones have obvious prebiotic syntheses and the primal role of nucleotide coenzymes has already been discussed. Secondly, membrane structure and the solubility differences between the electron and proton guarantee a link between electron and hydrogen ion transport fundamental to quantum symmetry-breaking. Electron transfer does not require the complex coded active sites required to catalyse specific molecular transformations. Model systems using Fe-porphyrins and imidazole can couple oxidative electron transport to phosphorylation (Brinigar et. al. 1966) and photo activated Mg-porphyrin to phosphate link (Goncharova and Goldfelt 1990, Lozovaya et. al. 1990).
A hint of how easily simple organic molecules can act by mutual catalysis to form or enhance energy production comes from the discovery that light-harvesting chlorophyll pigments enable mammalian mitochondria to capture photonic energy and produce ATP (Xu et al. 2014), probably by catalyzing the reduction of coenzyme Q, a slow step in mitochondrial ATP synthesis. Chlorophyll consists of an Mg2+- porphyrin with a phytol side chain, and porphyrin has prebiotic status, as already noted.

Fig 33: The role of chemiosmotic ion transport, redox electron transport and FeS centres (Lane and Martin).
A variety of geochemical settings for the origin of life have been proposed, including hydrothermal vents (Baross and Hoffman 1985), pyrite formation (Wachtershauser 1988, 2000) and catalytic FeS membranes (Russell et al. 1993), but Lane and Martin (2012 see also Lane 2009 and Martin and Russel 2007) provide compelling reasons to favor alkaline hydrothermal vents as the most likely site of the transition from geochemistry to life, based on their sustained far-from-equilibrium conditions and their basic similarities with the carbon and energy metabolism of autotrophic cells.
At Lost City, the exhalate precipitates into large spires (<60 m) of microporous minerals consisting of calcium magnesium carbonate. The thin mineral walls thereof (100 nm to 5 mm in diameter) form osmotic barriers that separate warm H2-rich alkaline fluids from cooler, more oxidized ocean waters (Kelley et al. (2001, 2005). Reduced, warm, alkaline fluids percolate continually through the labyrinths of micropores, sustaining thermal, redox, and pH gradients within the vents. Secondary convection in the adjacent ocean waters guarantees a steady supply of CO2 and other solutes to the mound's margins. At the interface with Fe2+-containing oceans (Arndt and Nisbet, E. 2012), the hydrothermal mounds on the early Earth would not have been carbonate spires as at Lost City today but would have been rich in transition metal sulfides instead.
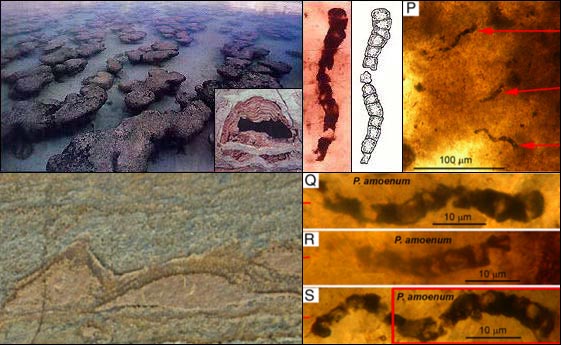 Fig 34: Modern stromatolites (above left), structures built of cyanobacteria (blue-green algae) grace Shark Bay, Australia. J. William Schopf (Scientific American Feb 1991) found remnants of 3.6 billion-year-old stromatolites (inset) lying near fossils of 3.5 billion-year-old cells (centre right) that resemble modern cyanobacteria (Schopf 1993), with strings of microscopic cells (centre right). Schopf et al. (2017) analysed these rocks in greater chemical detail by secondary ion mass spectroscopy (SIMS) from a thin section of the 3.465 billion year old Apex chert of northwestern Western Australia, and show their δ13C compositions vary systematically taxon to taxon from 31% to 39%. The SIMS data for the two highest δ13C Apex taxa are consistent with those of extant phototrophic bacteria; those for a somewhat lower δ13C taxon, with nonbacterial methane-producing Archaea; and those for the two lowest δ13C taxa, with methane-metabolizing γ-proteobacteria. The diversity of these, consistent with the existent tree of life, imply life must have originated 500 million years earlier or around 4 billion years ago. Allen Nutman and co-workers (Nutman et al. 2016) also discovered putative stromatolites (lower left) dating to 3.7 billion years in the Isua formation, in Greenland, however subsequent analysis of these have assigned their formation to tectonic processes (Allwood et al. 2018), which are believed to have begun 3.2-3.7 billion years ago (Brenner et al. 2020, Roderink et al. 2021). Life thus appears to have arisen within the first few hundred million years of Earth's formation from the planetary disc.
Fig 34: Modern stromatolites (above left), structures built of cyanobacteria (blue-green algae) grace Shark Bay, Australia. J. William Schopf (Scientific American Feb 1991) found remnants of 3.6 billion-year-old stromatolites (inset) lying near fossils of 3.5 billion-year-old cells (centre right) that resemble modern cyanobacteria (Schopf 1993), with strings of microscopic cells (centre right). Schopf et al. (2017) analysed these rocks in greater chemical detail by secondary ion mass spectroscopy (SIMS) from a thin section of the 3.465 billion year old Apex chert of northwestern Western Australia, and show their δ13C compositions vary systematically taxon to taxon from 31% to 39%. The SIMS data for the two highest δ13C Apex taxa are consistent with those of extant phototrophic bacteria; those for a somewhat lower δ13C taxon, with nonbacterial methane-producing Archaea; and those for the two lowest δ13C taxa, with methane-metabolizing γ-proteobacteria. The diversity of these, consistent with the existent tree of life, imply life must have originated 500 million years earlier or around 4 billion years ago. Allen Nutman and co-workers (Nutman et al. 2016) also discovered putative stromatolites (lower left) dating to 3.7 billion years in the Isua formation, in Greenland, however subsequent analysis of these have assigned their formation to tectonic processes (Allwood et al. 2018), which are believed to have begun 3.2-3.7 billion years ago (Brenner et al. 2020, Roderink et al. 2021). Life thus appears to have arisen within the first few hundred million years of Earth's formation from the planetary disc.
THE LAST UNIVERSAL COMMON ANCESTOR
We now turn to the earliest evidence of the evolutionary tree of life (King 2009). Recent research suggests that the last universal common ancestor (LUCA) of all life on the planet may have arisen from a phase interface between alkaline hydrogen-emitting undersea vents and the archaic acidified iron-rich ocean (Martin and Russel 2003), giving rise to an active iron-sulphur reaction phase still present in living cells and associated with electron transport and some of the most ancent proteins, such as ferredoxin, in which differential dynamics in membranous micropores in the vents managed to concentrate polypeptides and polynucleotides to biologically sustainable levels (Baaske et. al. 2007, Budin et. al. 2009), giving rise to the RNA era, while at the same time providing a free energy source based on proton transport across membranous microcellular interfaces resulting from fatty acids also being concentrated above their critical aggregate concentration.
Fig 35: Proposed scheme for the universal common ancestor. (Martin & Russell 2003).
The universal common ancestor of the three domains of life, archaea, prokaryotes and eucaryotes, may have thus been a proton-pumping membranous interface from which archaea and bacteria emerged as free-living adaptions. This is suggested by fundemantal differences in their cell walls and other details of evolutionary relationships among some of the oldest genes.
It has also been proposed, on the basis of the highly-conserved commonality of transcription and translation proteins to all life, but the apparently independent emergence of distinct DNA replication enzymes in archaea/eucaryotes and eubacteria, that the last universal common ancestor had a mixed RNA-DNA metabolism based on reverse transcriptase, pinpointing it to the latter phases of the RNA era (Leipe et. al. 1999).
In a ground-breaking project to identify genes that can illuminate the biology of LUCA, a team associated with Martin, (Weiss et al. 2016) took a phylogenetic approach to decoding the LUCA metabolism. Among proteins encoded in sequenced prokaryotic genomes, they sought those that: (1) are present in at least two higher taxa of bacteria and archaea, and (2) its tree should recover bacterial and archaeal monophyly. Genes meeting both criteria are unlikely to have undergone transdomain lateral gene transfer (LGT), and thus were probably present in LUCA and inherited within domains since then. By focusing on phylogeny rather than universal gene presence, they identified genes involved in LUCA's physiology - the ways that cells access carbon, energy and nutrients from the environment for growth.
The presence of the thermophile-specific enzyme reverse gyrase implies that LUCA was a thermophile. A rotator-stator ATP synthase subunit suggests LUCA was able to harness ion gradients for energy. LUCA also appears to have had a gene for a 'revolving door' protein that could swap sodium and hydrogen ions across this gradient. Earlier studies by Martin and Nick Lane of University College London suggest that such a protein would have been absolutely crucial for exploiting the natural gradient at vents. Cells conserve energy via chemiosmotic coupling with rotor - stator-type ATP synthases or via substrate-level phosphorylation. LUCA's genes encompass both a phosphotransacetylase (PTA) and an ATP synthase subunit. PTA generates acetylphosphate from acetyl-CoA, conserving the energy in the thioester bond, which can phosphorylate ADP or other substrates.
The only energy pathway enzymes present were those of the Wood-Ljungdahl (WL) pathway, which uses H2 as an electron donor and CO2 as electron acceptor. LUCA's WL enzymes are replete with FeS and FeNiS centres, indicating transition-metal requirements and requiring organic cofactors: flavin, F420, methanofuran, two pterins (the molybdenum cofactor MoCo and tetrahydromethanopterin) and corrins such as cobalamin (fig 27), as well as nucleotide and other cofactors. The nitrogenase molybdenum-iron protein alpha and beta chains, as well as NifH, indicate active nitrogen fixation, supporting the LUCA hypothesis (Wachtershauser 1988b, Leigh 2000, Raymond et al. 2004, Boyd & Peters 2013). This implicates the FeS-Mo centre of nitrogenase as well as the FeS-Ni centre of CO-methylating acetyl-CoA synthase as having a primordial catalytic geochemical status.
The H2 must have come from geological sources, since it could not have been made through fermentation. Analysis of the phylogenetic trees constructed from the 355 protein families places Clostridia and methanogens as the earliest-diverging organisms - both of which are anaerobic, H2 -dependent and use the WL pathway. In methanogens and acetogenic clostridia, methyl groups are central to growth, comprising the very core of carbon and energy metabolism. The implication of this work is that LUCA was very much dependent on abiotic sources of H2 to provide it with energy, consistent with a metabolism associated with lost-city vents in which alkaline mineral rich water enters the acidic high-CO2 ocean (fig 24).
 Fig 35b: Elements of LUCA's metabolism elucidated in Weiss et al. (2016). (a) The overall metabolic pathways iin LUCA. CODH/ACS, carbon monoxide dehydrogenase/acetyl CoA-synthase; Nif, nitrogenase; GS, glutamine synthetase; Mrp, MrP type Na+/H+ antiporter; CH3-R, methyl groups; HS-R, organic thiols. (b) Prominent methyl groups, and S and Se modifications. (c) Methyl transfer from tetrahydrofolate to methane and other C-containing biomolecules. (d) Molybdenum-containing MoCo. (e) SAM S-adenosyl-methionine attached to an FeS center. (f) The WL pathway showing how electron transfer from H2 to CO2 enables incorporation of metabolic molecules.
Fig 35b: Elements of LUCA's metabolism elucidated in Weiss et al. (2016). (a) The overall metabolic pathways iin LUCA. CODH/ACS, carbon monoxide dehydrogenase/acetyl CoA-synthase; Nif, nitrogenase; GS, glutamine synthetase; Mrp, MrP type Na+/H+ antiporter; CH3-R, methyl groups; HS-R, organic thiols. (b) Prominent methyl groups, and S and Se modifications. (c) Methyl transfer from tetrahydrofolate to methane and other C-containing biomolecules. (d) Molybdenum-containing MoCo. (e) SAM S-adenosyl-methionine attached to an FeS center. (f) The WL pathway showing how electron transfer from H2 to CO2 enables incorporation of metabolic molecules.
LUCA's genes for RNA nucleoside modification indicate that it performed chemical modification of nucleosides in both tRNA and rRNA. Four of LUCA's nucleoside modifications are methylations requiring SAM. In the modern code, several base modifications are required for codon-anticodon interactions at the wobble position. Consistent with the recurrent role of methyl groups in LUCA's biology, by far the most common tRNA and rRNA nucleoside modifications that are conserved across the archaeal bacterial divide are methylations, although thio-methylations and incorporation of sulfur and selenium are observed. This picture indicates the antiquity and functional significance of methylated bases in the evolution of the ribosome and the genetic code and forges links between the genetic code, primitive carbon and energy metabolism and hydrothermal environments. Notably selenophosphate synthase is included in the LUCA list implicating primal dependence on selenium-catalysed transformations, and the likely inclusion of selenocystine in the primal genetic code. The SECIS loop structure added to mRNAs to edit the genetic code in read-time to include seleno-cysteine is also common to all forms of life indicating its presence in LUCA.
Lane 2010 has an interesting discussion of the role of H+ gradients in both the rotating ATPase, fig 30, and the leverages in the respiration transport pipeline (Efremov et al. 2010). Phylometabolic reconstructions (Braakman & Smith 2012) as well as chemical experiments suggest the Wood-Ljungdahl pathway may have prebiotic origins (Varma et al. 2018). The structure of the acetyl-CoA synthase is elucidated in Hegg (2004).
Models of the Hadean atmosphere around 3.8 billion years ago, suggest abiotic nitrogen fixation was active in the early high CO2 atmosphere and only declined by 2.2 billion years ago (Navarro-Gonzalez et al. 2001). Sulphur isotope ratios indicate life was thriving by 3.5 billion years ago (Sim et al. 2019). The rapid growth of genetic diversity in the Archaean 3.4-3.0 billion years ago (David and Alm 2010) puts LUCA into the zone between 3.8 and 3.4. This indicates that the supply of prebiotic HCN and O-containing molecules was sufficient to support biogenesis, driven by the ongoing energy interface of Lost City vents for some 400 million years until life had become established, but that the Archaean nitrogen crisis had set in before LUCA had diverged into the archaea and bacteria. It is also possible that the primordial Mo-FeS nitrogenase first evolved as a cyanide detoxifying enzyme, as the molybdenum-nitrogenase is superior in reducing cyanide (Postgate 1974).
LUCA's gene list reveals only nine nucleotide biosynthesis and five amino acid biosynthesis proteins. The paucity of enzymes for essential amino acid, nucleoside and cofactor biosyntheses suggests that LUCA might not yet have evolved the genes in question prior to the bacterial-archaeal split, with the pathway products for LUCA being still provided by primordial geochemistry.


Fig 35c: Left: (A) Bacterial ferredoxin's asymmetric sequence (B) Structural alignment of N and C structures showing structural symmetry of the parent protein. (C) Unrooted maximum-likelihood phylogenetic tree of N-terminal motif (red) and C-terminal motif (blue). (D) Color block diagrams of asymmetric vs. symmetric designs. (E) Evolutionary scheme.Right: Bacterial ferredoxin and a putative ancestral molecule of both ferredoxins and flavodoxins (Raanan et al. 2020).
Using computer models Mutter A et al. (2019) designed ancestral forms of the FeS protein ferredoxin that may have existed at an earlier stage in the evolution of life. That research led to the creation of a basic version of the protein -- a simple ferredoxin consisting of two symmetric components that is able to conduct electricity within a cell and that, over eons of evolution, could have given rise to the many asymmetric types of ferredoxin that exist today, starting from a single FeS core. They took the genome of E. coli, removed the gene it uses to create ferredoxin in nature, and spliced in a gene for their reverse-engineered protein. The modified E. coli colony survived and grew although more slowly than normal. Raanan et al. (2020) have followed this with a putative ancestor to both ferredoxins and flavodoxins.
The late heavy bombardment (LHB) of Earth by comets and asteroids approximately 4-3.8 billion years ago probably resulted in Earth being periodically heated to the point that the oceans were vaporized and probably led to bottlenecks in the diversity of life at the time, meaning that only hyperthermophiles survived. The amount of oxygen available for biological cells was negligible and all life was anaerobic. When we look at the inferred metabolism of LUCA, we are looking at the dominant and most successful kind of metabolism on the planet before the Bacteria and Archaea diverged.
 Fig 36: Hypothetical branching and evolution of RNA and DNA replication machinery (Leipe et. al. 1999).
Fig 36: Hypothetical branching and evolution of RNA and DNA replication machinery (Leipe et. al. 1999).
THE PRECOCIOUS ORIGINS OF LIFE ON EARTH
Far from being an improbable accident taking billions of years to find the right conditions, life appears to have become established on Earth as soon as the conditions permitted a liquid water ocean, suggesting either that Earth was richly bombarded with complex organic molecules which quickly found within the diversity of microclimates on Earth some which were directly conducive to the processes leading the to the genetic epoch, or that life's had already begun in the gas and dust cloud initially forming the solar system.
The severity of the Hadean epoch beginning around 4.6 bya has been questioned, by studies estimating that after the first few hundred million years of bombardment, the heat from asteroid impacts had dissipated enough that 10 to 75 percent of the top kilometer of the subsurface was habitable for mesophiles – microbes living in temperatures of 20° to 50° Celsius (Grimm & Marchi 2018). The influx of asteroids also diminished over thefirst 150 million years and even with a possible Late Heavy Bombardment the habitable zone would not have been destroyed.
 Fig 36b: Possible graphite inclusions suggesting an origin 3.85-3.95 bya. Left: Graphite inclusions show evidence for biogenic disordered graphite, signal inset (Mojzsis et. al 1996). Right: Graphite grains from Labrador (Tashiro et al. 2017).
Fig 36b: Possible graphite inclusions suggesting an origin 3.85-3.95 bya. Left: Graphite inclusions show evidence for biogenic disordered graphite, signal inset (Mojzsis et. al 1996). Right: Graphite grains from Labrador (Tashiro et al. 2017).
In the Jack Hills of Western Australia detrital igneous zircons with U-Pb crystallization ages as old as 4.4 billion years occur in Archean clastic sediments deposited at ~3 billion years ago. The most recent discovery of disordered graphite inclusions of zircons from Western Australia, with a high 12C content, consistent with a biogenic origin, that formed 4.1 billion tears ago (Bell et al. 2015), pushing the earliest date for the origin of life back 300 million years from previous estimates, suggests life was prevalent enough before then to become included in the geological record. This date is highly significant, since the oldest direct evidence for the presence of surface waters are slightly younger ca. ~3.8 billion years old sedimentary rocks called banded iron formation (BIF) that are exposed at Isua in southwest Greenland.
Gustaf Arrhenius, (Mojzsis et. al 1996.) studying tiny apatite grains in the Isua formation of Greenland, has found carbon 12 to 13 ratios consistent with the grains originating from living matter. The Isua rocks date from 3.85 billion years ago. Although indications from zircon crystals indicate a solid crust 4.2 billion years ago, no intact rocks have been discovered older than 3.96 billion years. The moon and probably the Earth likewise was heavily bombarded with meteors up to 3.8 billion years ago, suggesting that life evolved on earth as soon as environmental conditions allowed. Tashiro T et al. (2017) further report analysing carbon isotopes in powdered rock and in individual graphite grains from the Saglek area, Labrador, Canada. Uranium-lead dating on ancient zircon crystals inside a type of metamorphic rock called gneiss in the Saglek block to indicate that the gneiss was 3.95 billion years old. The researchers claim that the graphite containing hints of life must be at least that old, because it lies within rocks that include the 3.95-billion-year-old gneiss. Labrador's ancient rocks, including the Saglek block, lie directly across the Labrador Sea from southwestern Greenland, where the Isua find occurred..
There is continuing debate about whether the former chemical and 'fossil' traces, now further studied with Raman spectroscopy to give carbon isotope evidence, really represent early cyanobacterial life, prebiotic 'soup' or volcanic or meteorite material (Schopf et. al. 2002, Brazier et. al. 2002, Mojzsis 2002). However some researchers contend on the basis of inorganic simulations that these microfossils are purely mineral [Hogan 2003]. Jacques Touret [2003] has found that methane as well as high salt water trapped in pillow lava from Isua suggesting the involvement of hydrothermal vents beside an undersea volcano. However these findings are questioned by David Vanko (Necht 2003). John Parnell has also suggested radioactivity trapped in oily grains may have had a role (Lawton 2003).
Nevertheless, there is consensus agreement that life was under way by 3.5 billion years the age of the fossil stromatolite in fig 34, although the nature of these is also debated. These fossils could be the earliest evidence of life on Earth, yet these relics, with names like Chromoccoceae and Oscdlotorioceoe, are morphologically identical to modern cyanobacteria that cover the globe from Antarctica to the Sahara [Cohen 1996]. In July 2011 Brazier who had questioned the biological nature of the earliest fossils, himself claims to have discovered a genuine biological fossil of sulphur bacteria dating to 3.43 billion years (Wacey et al. 2011). New techniques are also emerging (Brazier et al. 2015).
Fig 37:  a,b,e, Clusters of cells, some showing cell wall rupturing (arrows in a,b), folding or invagination (arrow in e). c,d,h, Chains of cells with cellular divisions (arrows). f,i-j, Cells attached to detrital quartz grains, exhibiting cell walls (Wacey et al. 2011).
a,b,e, Clusters of cells, some showing cell wall rupturing (arrows in a,b), folding or invagination (arrow in e). c,d,h, Chains of cells with cellular divisions (arrows). f,i-j, Cells attached to detrital quartz grains, exhibiting cell walls (Wacey et al. 2011).
The oldest compelling fossil evidence for cellular life dates back to 3.45 billion years ago. Schopf et al. (2017) have analysed fossil cell forms first discovered 20 years ago (Schopf 2003) and found cells with carbon isotope ratios consistent with a diverse ecology of cyanobacteria, methanogenic archaea and methame-metabolizing γ-proteobacteria in 3.465 billion year old Apex chert of northwestern Western Australia, implying an origin for life around 4 billion years ago (fig 34 ).The researchers commented: "The evidence that a diverse group of organisms had already evolved extremely early in the Earth's history strengthens the case for life existing elsewhere in the Universe because it would be extremely unlikely that life formed quickly on Earth but did not arise anywhere else.".
Similar evidence has been discovered on a 3.43-billion-year old beach in western Australia. Its grains of sand provided a home for cells that dined on sulphur in a largely oxygen-free world (O'Donaghue 2011). The origins of the first fossil life forms including the stromatolites in fig 34 (Nutman et al. 2016), likewise lie at the limits of the geological record. At around 3.5 billion years old, fossilised bacteria are the earliest evidence of life on Earth, and yet these relics, with names like Chromoccoceae and Oscdlotorioceoe, are morphologically identical to the sophisticated modern cyano-bacteria that cover the globe from Antarctica to the Sahara (Cohen 1996).
 Perhaps another milestone of 3.77 - 4.28 billion years has been found in terms of tubes, carbonate rosettes and other structures in ferruginous sedimentary rocks, interpreted as seafloor-hydrothermal vent-related precipitates, from the Nuvvuagittuq belt in Quebec, Canada. These also have high C-12 ratios consistent with a biotic origin (Dodd et al. 2017).
Perhaps another milestone of 3.77 - 4.28 billion years has been found in terms of tubes, carbonate rosettes and other structures in ferruginous sedimentary rocks, interpreted as seafloor-hydrothermal vent-related precipitates, from the Nuvvuagittuq belt in Quebec, Canada. These also have high C-12 ratios consistent with a biotic origin (Dodd et al. 2017).
Fig 37b: Carbon tube structures from Dodd et al. (2017).
Evidence for the emergence of the eucaryotes that lead to the higher organisms also gpes back over 2 billion years ago. Traces of oil extracted from Australian shale have pushed the date for the origin of complex cells back another half a billion years. Compounds in the oil suggest that eucaryotic cells, which make up all life on Earth except for bacteria, had evolved as early as 2.7 billion years ago, however this conclusion has been refuted leading to a date range of 2.1-1.5 Bya (French et al. 2015). It is not until about 2.1 billion years ago that fossil imprints appear in the geological record that are so large that they can only be eucaryotes. A team of researchers in Australia has found steranes, molecules with 26 to 30 carbon atoms arranged in four rings, in droplets of oil extracted from rock 700 metres below the surface in the Pilbara region of north-western Australia. These are produced by the decay of cholesterol and other steroids found in the membranes of eukaryotes, but not bacteria (Brocks et. al.). Genetic analysis of the base of the tree of life indicates the oldest branches of both archaea and (eu)bacteria are thermophilic suggesting a period in hot pools or significant meteoric impact leaving only the thermophiles as survivors.
There is also potential evidence from incoming meteorites believed t have source from Mars of life having existed on Mars. The ALH-77005 Martian meteorite was found in Allan Hills on Antarctica during the Japanese National Institute of Polar Research (1977-1978) mission. Biosignatures were determined by 1) coccoidal, filamentous forms, 2) presence of embedded organic material, 3) presence of biogenic minerals, like ferrihydrite, goethite, and hematite. The other signatures for biogenicity of this meteorite are strong negative δ13C, enrichment of Fe, Mn, P, Zn in shock melt support the scenario (Gyollai 2019).
The Copernican principle asserts that the Earth is a typical rocky planet in a typical planetary system, located in an unexceptional region of a common barred-spiral galaxy, hence it is probable that the universe teems with complex life. This is supported to a reasonable extent by the discovery of an increasing number of planets including some putative "Goldilocks" zone planets where water would be liquid and life as we know it could potentially exist. Set against this, the Rare Earth hypothesis argues that the emergence of complex life requires a host of fortuitous circumstances including a galactic habitable zone, a central star and planetary system having the requisite character, the circumstellar habitable zone, the size of the planet, the advantage of a large satellite, conditions needed to assure the planet has a magnetosphere and plate tectonics, the chemistry of the lithosphere, atmosphere, and oceans, the role of "evolutionary pumps" such as massive glaciation and rare bolide impacts, and whatever led to the still mysterious Cambrian explosion of animal phyla. This might mean that planets able to support a bacterial level of life are not so uncommon, but those supporting complex multicellular life might be.
Bringing this question to a pivotal crux in our context, the emergence of mitochondria as endosymbionts has been proposed to be a critical bottleneck which allowed complex life to evolve only once, because, only in this effectively fractal cellular architecture, can the membrane surface areas necessary to support the chemical reactions enabling the vastly larger number of genes in a complex organism's genome to maintain metabolic stability (Lane and Martin The energetics of genome complexity 2010 doi:10.1038doi:10.1038/nature09486). Whether such endo-symbiosis is rare. or a common extreme of parasitic relationships would then determine how likely or unlikely complex life might be.
Offset against both the uniqueness of the mitochondrial endo-symbiosis and the closely linked, but independent question of the origin of the nucleus and nuclear envelope, has been the discovery of mimiviruses and mamaviruses infecting amoeba (Raoult et, al. The 1.2-Mb Genome Sequence of Mimivirus doi: 10.1126/science.1101485) and related very large aquatic viruses such as CroV infecting single celled plankton species, which despite their recent discovery, appear from ocean gene analyses to be potentially ubiquitous and widespread in the oceans and possibly playing a crucial role in regulating the atmospheric-oceanic pathways, such as carbon sequestration (Fisher, Allen, Wilson and Suttle 2010 Giant virus with a remarkable complement of genes infects marine zooplankton PNAS doi: 10.1073/pnas.1007615107). These form an intermediate genetic position between viruses and cells, having the largest genomes, with extensive cellular machinery and larger than the smallest completely autonomous bacterial and archaeal genomes.
Mimiviruses also host parasitic virophages, affectionately named sputnik as viral satellites, which piggy back on the metabolism of the large viral factories set up by these giant viral genomes causing the mimiviruses to sicken, and these virophages also contains genes that are linked to viruses infecting each of the three domains of life Eukarya, Archaea and Bacteria (La Scola et. al. The virophage as a unique parasite of the giant mimivirus Nature doi:10.1038/nature07218). It has thus been suggested that they have a primary role in the establishment of cellular life and that they may have been instrumental in the emergence of the nuclear envelope.
On to: Tree of Life: Tangled Roots and Sexy Shoots
Evolutionary radiation from the last universal common ancestor of all life to Homo sapiens.

Fig 38: A quadratic iteration compared with the interactive effects of inverse quadratic charge interaction. The fractal structures of tissues have features similar to the Mandelbrot set on changes of scale. The fractal effects reach from the molecular (a) in which individual proteins are illustrated embedded in the lipid membrane, through cell organelles (b) to the intercellular structure of whole organs as illustrated by skin (c). Such scale-dependent coherence of structure is possible only because of the highly non-linear nature of the electromagnetic force in quantum charge interactions of fermionic matter (Campbell).
On to: